当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Tailor-Made Deazaflavin-Mediated Recycling System for Artificial Nicotinamide Cofactor Biomimetics
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acscatal.1c03033 Jeroen Drenth 1 , Guang Yang 1 , Caroline E Paul 2 , Marco W Fraaije 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acscatal.1c03033 Jeroen Drenth 1 , Guang Yang 1 , Caroline E Paul 2 , Marco W Fraaije 1
Affiliation
|
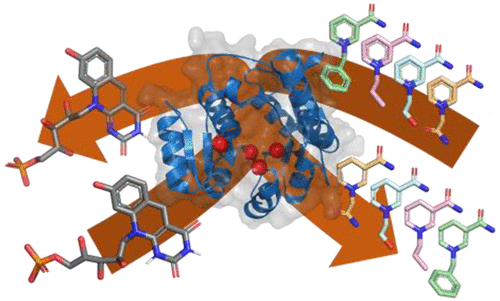
Nicotinamide adenine dinucleotide (NAD) and its 2′-phosphorylated form NADP are crucial cofactors for a large array of biocatalytically important redox enzymes. Their high cost and relatively poor stability, however, make them less attractive electron mediators for industrial processes. Nicotinamide cofactor biomimetics (NCBs) are easily synthesized, are inexpensive, and are also generally more stable than their natural counterparts. A bottleneck for the application of these artificial hydride carriers is the lack of efficient cofactor recycling methods. Therefore, we engineered the thermostable F420:NADPH oxidoreductase from Thermobifida fusca (Tfu-FNO), by structure-inspired site-directed mutagenesis, to accommodate the unnatural N1 substituents of eight NCBs. The extraordinarily low redox potential of the natural cofactor F420H2 was then exploited to reduce these NCBs. Wild-type enzyme had detectable activity toward all selected NCBs, with Km values in the millimolar range and kcat values ranging from 0.09 to 1.4 min–1. Saturation mutagenesis at positions Gly-29 and Pro-89 resulted in mutants with up to 139 times higher catalytic efficiencies. Mutant G29W showed a kcat value of 4.2 s–1 toward 1-benzyl-3-acetylpyridine (BAP+), which is similar to the kcat value for the natural substrate NADP+. The best Tfu-FNO variants for a specific NCB were then used for the recycling of catalytic amounts of these nicotinamides in conversion experiments with the thermostable ene-reductase from Thermus scotoductus (TsOYE). We were able to fully convert 10 mM ketoisophorone with BAP+ within 16 h, using F420 or its artificial biomimetic FOP (FO-2′-phosphate) as an efficient electron mediator and glucose-6-phosphate as an electron donor. The generated toolbox of thermostable and NCB-dependent Tfu-FNO variants offers powerful cofactor regeneration biocatalysts for the reduction of several artificial nicotinamide biomimetics at both ambient and high temperatures. In fact, to our knowledge, this enzymatic method seems to be the best-performing NCB-recycling system for BNAH and BAPH thus far.
中文翻译:

定制的脱氮黄素介导的人工烟酰胺辅因子仿生回收系统
烟酰胺腺嘌呤二核苷酸 (NAD) 及其 2'-磷酸化形式 NADP 是大量具有重要生物催化作用的氧化还原酶的关键辅助因子。然而,它们的高成本和相对较差的稳定性使得它们对于工业过程的电子介体不太有吸引力。烟酰胺辅因子仿生剂 (NCB) 易于合成、价格低廉,而且通常比天然对应物更稳定。这些人造氢化物载体应用的瓶颈是缺乏有效的辅因子回收方法。因此,我们通过结构启发的定点诱变,从Thermobifida fusca ( Tfu- FNO) 中设计了热稳定的 F 420 :NADPH 氧化还原酶,以适应八个 NCB 的非天然 N1 取代基。然后利用天然辅因子 F 420 H 2的极低氧化还原电位来还原这些 NCB。野生型酶对所有选定的 NCB 均具有可检测的活性, K m值在毫摩尔范围内, k cat值在 0.09 至 1.4 min –1范围内。 Gly-29 和 Pro-89 位点的饱和诱变产生的突变体催化效率高出 139 倍。突变体 G29W 对 1-苄基-3-乙酰基吡啶 (BAP + ) 显示出 4.2 s –1的k cat值,这与天然底物 NADP + 的k cat值相似。 然后,在使用来自Thermus scotoductus ( Ts OYE)的热稳定烯还原酶的转化实验中,将针对特定NCB的最佳Tfu- FNO变体用于回收这些烟酰胺的催化量。使用 F 420或其人工仿生 FOP (FO-2'-磷酸盐) 作为有效的电子介体和葡萄糖-6-磷酸盐作为电子供体,我们能够在 16 小时内用 BAP +完全转化 10 mM 酮异佛尔酮。生成的热稳定性和 NCB 依赖性Tfu -FNO 变体工具箱提供了强大的辅因子再生生物催化剂,可在环境温度和高温下还原几种人造烟酰胺仿生剂。事实上,据我们所知,这种酶法似乎是迄今为止 BNAH 和 BAPH 性能最好的 NCB 回收系统。
更新日期:2021-09-17
中文翻译:

定制的脱氮黄素介导的人工烟酰胺辅因子仿生回收系统
烟酰胺腺嘌呤二核苷酸 (NAD) 及其 2'-磷酸化形式 NADP 是大量具有重要生物催化作用的氧化还原酶的关键辅助因子。然而,它们的高成本和相对较差的稳定性使得它们对于工业过程的电子介体不太有吸引力。烟酰胺辅因子仿生剂 (NCB) 易于合成、价格低廉,而且通常比天然对应物更稳定。这些人造氢化物载体应用的瓶颈是缺乏有效的辅因子回收方法。因此,我们通过结构启发的定点诱变,从Thermobifida fusca ( Tfu- FNO) 中设计了热稳定的 F 420 :NADPH 氧化还原酶,以适应八个 NCB 的非天然 N1 取代基。然后利用天然辅因子 F 420 H 2的极低氧化还原电位来还原这些 NCB。野生型酶对所有选定的 NCB 均具有可检测的活性, K m值在毫摩尔范围内, k cat值在 0.09 至 1.4 min –1范围内。 Gly-29 和 Pro-89 位点的饱和诱变产生的突变体催化效率高出 139 倍。突变体 G29W 对 1-苄基-3-乙酰基吡啶 (BAP + ) 显示出 4.2 s –1的k cat值,这与天然底物 NADP + 的k cat值相似。 然后,在使用来自Thermus scotoductus ( Ts OYE)的热稳定烯还原酶的转化实验中,将针对特定NCB的最佳Tfu- FNO变体用于回收这些烟酰胺的催化量。使用 F 420或其人工仿生 FOP (FO-2'-磷酸盐) 作为有效的电子介体和葡萄糖-6-磷酸盐作为电子供体,我们能够在 16 小时内用 BAP +完全转化 10 mM 酮异佛尔酮。生成的热稳定性和 NCB 依赖性Tfu -FNO 变体工具箱提供了强大的辅因子再生生物催化剂,可在环境温度和高温下还原几种人造烟酰胺仿生剂。事实上,据我们所知,这种酶法似乎是迄今为止 BNAH 和 BAPH 性能最好的 NCB 回收系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号