Critical Care ( IF 8.8 ) Pub Date : 2021-09-03 , DOI: 10.1186/s13054-021-03741-z Carol L Hodgson 1, 2 , Stefan J Schaller 3, 4, 5 , Peter Nydahl 6 , Karina Tavares Timenetsky 7 , Dale M Needham 8, 9
In the last decade, there have been more than 40 randomized trials evaluating early mobilization and rehabilitation in intensive care units (ICU) [1]. Such trials generally aim to reduce the incidence of ICU-acquired weakness (ICUAW) which is associated with poor long-term survival, physical functioning, and quality of life [2]. At least eight international guidelines have recommended ICU early mobilization and rehabilitation [3].
Despite supporting evidence and guidelines, implementation of ICU mobilization and rehabilitation is highly variable[4]. Hence, we report on 10 steps to help ICU clinicians in optimizing early mobilization and rehabilitation.
Early mobilization and rehabilitation is more successful in ICUs with a culture that prioritizes and values this intervention [5]. Mobility champions can help develop this culture using leadership and communication skills to educate, train, coordinate, and promote patient mobilization [3, 4, 6]. They support staff with an emphasis on safety and practical skills to improve the team’s confidence and capabilities [6].
A structured QI approach can greatly enhance successful implementation of early mobilization and rehabilitation [7]. One approach to QI includes four steps: (1) summarizing the evidence; (2) identifying barriers (e.g., sedation or lack of equipment); (3) establishing performance measures (e.g., sedation targets, frequency, and level of patient mobilization); and (4) ensuring all eligible patients receive the intervention (via appropriate engagement, education, execution, and evaluation) [6, 7].
A systematic review identified 28 unique barriers to early mobilization and rehabilitation, including patient-related barriers (e.g., physiological instability and medical devices), structural barriers (e.g., limited staff and equipment), procedural barriers (e.g., lack of coordination and delayed screening for eligibility), and cultural barriers (e.g., prior staff experience and ICU priorities for patient care) [4]. There are many strategies to effectively overcome barriers, including implementation of safety guidelines; use of mobility protocols; interprofessional training, education, and rounds; and inclusion of physician champions [4].
The multi-professional team effort required for early mobilization and rehabilitation program depends on optimal communication. We recommend that interprofessional communication is facilitated using a structure adapted to the individual ICU that allows (algorithm-based) mobilization goals, including an opportunity for all team members to raise concerns and ensure flow of information regarding mobility goals and achievement across staff and over time [8].
ICU patients’ experience with early mobilization and rehabilitation is variable. It may be tiring, uncomfortable and difficult, while at other times be motivating and rewarding for patients [9]. With improving cognitive status, patients may be shocked by the severity of their muscle weakness. In the early stages of critical illness, patients may prefer to focus on short-time goals (e.g., sitting in a chair) set by the multidisciplinary team [9]. As patients progress, they may become more engaged in goal setting and longer-term rehabilitation planning (e.g., walking longer distances, sitting outside) (Fig. 1).
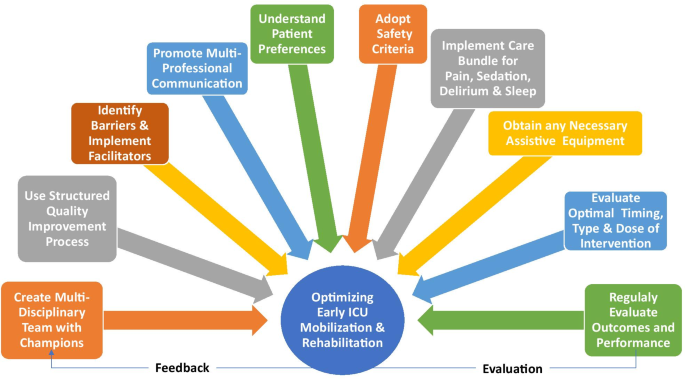
Ten strategies to optimize early mobilization and rehabilitation in ICU
Full size imageMeta-analyses have demonstrated the safety of in-bed and out-of-bed ICU mobilization, with rare occurrence of serious events [10]. One method of assessing safety is a traffic light system that provides specific criteria, across respiratory, hemodynamic, neurological, and other body systems, to be considered in mobilizing individual patients [11]. In this system, “red light” criteria indicate an increased potential for a serious safety event during mobilization requiring experienced decision-making, “yellow light” indicates potential risk that should be evaluated in terms of benefits versus risks, and “green light” indicates that mobilization is generally safe [11].
Patients’ sedation and delirium status is a common barrier to early mobilization and rehabilitation [4]. More broadly, pain, sedation, delirium, sleep, and early mobilization and rehabilitation are closely inter-related, as considered in clinical guidelines[3]. Assessment and management of these issues, via existing evidence-based practices (as synthesized in the guidelines), are important to maximize patients’ ability to participate in rehabilitation.
Barriers to early mobilization and rehabilitation may include ICUAW, impaired physical functioning, traumatic injuries, and obesity [6]. Equipment can expand treatment options, increase patient mobility and activity levels, and reduce risk of injury to staff [12]. Selecting rehabilitation equipment may be challenging, with important considerations including the equipment cost/availability, ability to share equipment between units or patients (including infection control considerations), and the physical space available for patient mobilization and for convenient storage of equipment. Evidence supporting use of specific equipment is still evolving, including evaluation of neuromuscular electrical stimulation (NMES), in-bed cycle ergometry, tilt tables, and other devices [12, 13].
Important knowledge gaps exist regarding exercise, including the timing, type, and dose of interventions. There is some evidence suggesting that starting rehabilitation within 2 or 3 days of ICU admission may be superior to later initiation [3]. Types of interventions to be considered include active functional mobilization, in-bed cycle ergometry, electrical muscle stimulation (with or without passive/active exercises), tilt tables, and use of various rehabilitation equipment. In addition, the intensity, duration, and frequency of each intervention type are important considerations [14]. Additional research is needed to further understand potential benefit or harm. Until that time, clinician judgement will play an important role and must be tailored to individual patients and to the dynamic nature of critical illness.
Mobility and rehabilitation-related measures, appropriate to the ICU setting and integrated into clinical care, are needed to set patient goals and track their progress, allocate scarce rehabilitation resources to those patients who may benefit the most, and conduct evaluations of structured quality improvement programs [15]. Understanding patients’ functioning prior to critical illness, and their own goals, are also important considerations.
Evidence is still evolving about early mobilization in ICU with ongoing large, multi-center trials. Further research is needed to understand the optimal timing, type and dose of interventions, and their effect on long-term patient outcomes. These 10 strategies provide guidance for implementing early mobilization and rehabilitation in the ICU with the goal of optimizing safety and effectiveness to improve patients’ experiences and outcomes.
Not applicable.
- 1.
Waldauf P, Jiroutkova K, Krajcova A, Puthucheary Z, Duska F. Effects of rehabilitation interventions on clinical outcomes in critically Ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2020;48(7):1055–65.
PubMed Google Scholar
- 2.
Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–83.
Article Google Scholar
- 3.
Lang JK, Paykel MS, Haines KJ, Hodgson CL. Clinical practice guidelines for early mobilization in the ICU: a systematic review. Crit Care Med. 2020;48(11):e1121–8.
Article Google Scholar
- 4.
Dubb R, Nydahl P, Hermes C, Schwabbauer N, Toonstra A, Parker AM, Kaltwasser A, Needham DM. Barriers and strategies for early mobilization of patients in intensive care units. Ann Am Thorac Soc. 2016;13(5):724–30.
Article Google Scholar
- 5.
Hodgson C, Bellomo R, Berney S, Bailey M, Buhr H, Denehy L, Harrold M, Higgins A, Presneill J, Saxena M, et al. Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care (London, England). 2015;19:81.
Article Google Scholar
- 6.
Parry SM, Nydahl P, Needham DM. Implementing early physical rehabilitation and mobilisation in the ICU: institutional, clinician, and patient considerations. Intensive Care Med. 2018;44(4):470–3.
Article Google Scholar
- 7.
Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17(4):271–81.
Article Google Scholar
- 8.
Schaller SJ, Anstey M, Blobner M, Edrich T, Grabitz SD, Gradwohl-Matis I, Heim M, Houle T, Kurth T, Latronico N, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388(10052):1377–88.
Article Google Scholar
- 9.
Corner EJ, Murray EJ, Brett SJ. Qualitative, grounded theory exploration of patients’ experience of early mobilisation, rehabilitation and recovery after critical illness. BMJ Open. 2019;9(2):e026348.
Article Google Scholar
- 10
Nydahl P, Sricharoenchai T, Chandra S, Kundt FS, Huang M, Fischill M, Needham DM. Safety of patient mobilization and rehabilitation in the intensive care unit. Systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766–77.
Article Google Scholar
- 11.
Hodgson CL, Stiller K, Needham DM, Tipping CJ, Harrold M, Baldwin CE, Bradley S, Berney S, Caruana LR, Elliott D, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit care (London, England). 2014;18(6):658.
Article Google Scholar
- 12.
Darragh AR, Campo MA, Frost L, Miller M, Pentico M, Margulis H. Safe-patient-handling equipment in therapy practice: implications for rehabilitation. Am J Occup Ther. 2013;67(1):45–53.
Article Google Scholar
- 13.
Zayed Y, Kheiri B, Barbarawi M, Chahine A, Rashdan L, Chintalapati S, Bachuwa G, Al-Sanouri I. Effects of neuromuscular electrical stimulation in critically ill patients: a systematic review and meta-analysis of randomised controlled trials. Aust Crit Care. 2020;33(2):203–10.
CAS Article Google Scholar
- 14.
Paton M, Lane R, Paul E, Cuthburtson GA, Hodgson CL. Mobilization during critical illness: a higher level of mobilization improves health status at 6 months, a secondary analysis of a prospective cohort study. Crit Care Med. 2021;49(9):e860–9. https://doi.org/10.1097/CCM.0000000000005058.
CAS Article PubMed Google Scholar
- 15.
Parry SM, Remedios L, Denehy L, Knight LD, Beach L, Rollinson TC, Berney S, Puthucheary ZA, Morris P, Granger CL. What factors affect implementation of early rehabilitation into intensive care unit practice? A qualitative study with clinicians. J Crit Care. 2017;38:137–43.
Article Google Scholar
Download references
None.
Professor Carol Hodgson is supported by a National Health and Medical Research Council Investigator grant.
Affiliations
Australian and New Zealand Intensive Care Research Centre, School of Public Health and Preventive Medicine, Monash University, 3/553 St Kilda Rd, Melbourne, VIC, 3004, Australia
Carol L. Hodgson
Department of Intensive Care and Hyperbaric Medicine, The Alfred, Melbourne, VIC, Australia
Carol L. Hodgson
Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Berlin, Germany
Stefan J. Schaller
Department of Anesthesiology and Operative Intensive Care Medicine, Humboldt-Universität zu Berlin, Chariteplatz 1, Berlin, Germany
Stefan J. Schaller
Department of Anesthesiology and Intensive Care, School of Medicine, Technical University of Munich, Munich, Germany
Stefan J. Schaller
Nursing Research, Department of Anaesthesiology and Intensive Care Medicine, University Hospital of Schleswig-Holstein, Kiel, Germany
Peter Nydahl
Department of Critical Care, Hospital Israelita Albert Einstein, São Paulo, SP, Brazil
Karina Tavares Timenetsky
Outcomes After Critical Illness and Surgery (OACIS) Group, Division of Pulmonary and Critical Care Medicine, Department of Physical Medicine and Rehabilitation, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
Dale M. Needham
School of Nursing, Johns Hopkins University, Baltimore, MD, USA
Dale M. Needham
- Carol L. HodgsonView author publications
You can also search for this author in PubMed Google Scholar
- Stefan J. SchallerView author publications
You can also search for this author in PubMed Google Scholar
- Peter NydahlView author publications
You can also search for this author in PubMed Google Scholar
- Karina Tavares TimenetskyView author publications
You can also search for this author in PubMed Google Scholar
- Dale M. NeedhamView author publications
You can also search for this author in PubMed Google Scholar
Contributions
CLH and DMN conceived the manuscript, all authors were involved in the preparation, editing, and final review of the manuscript.
Corresponding author
Correspondence to Carol L. Hodgson.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
CH is a principal investigator on a NHMRC-funded, multi-center Phase III randomized trial evaluating early mobilization in intensive care (GNT1120319). DMN is a principal investigator on a NIH-funded, multi-centered randomized trial (R01HL132887) evaluating nutrition and exercise in acute respiratory failure. This trial has received an unrestricted research grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Devices. DMN has previously provided consulting to Haisco-USA Pharmaceuticals, Novartis Pharma (Switzerland), and GlaxoSmithKline (UK). For all other authors, there is no conflicts of interest related to this manuscript.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and Permissions
Cite this article
Hodgson, C.L., Schaller, S.J., Nydahl, P. et al. Ten strategies to optimize early mobilization and rehabilitation in intensive care. Crit Care 25, 324 (2021). https://doi.org/10.1186/s13054-021-03741-z
Download citation
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-021-03741-z
中文翻译:

优化重症监护早期活动和康复的十项策略
在过去十年中,已有 40 多项随机试验评估了重症监护病房 (ICU) 的早期活动和康复 [1]。此类试验通常旨在降低 ICU 获得性虚弱 (ICUAW) 的发生率,ICUAW 与长期生存率、身体机能和生活质量差有关 [2]。至少有八项国际指南推荐 ICU 早期活动和康复 [3]。
尽管有证据和指南支持,但 ICU 活动和康复的实施变化很大[4]。因此,我们报告了帮助 ICU 临床医生优化早期活动和康复的 10 个步骤。
在重视和重视这种干预的文化的 ICU 中,早期活动和康复更成功 [5]。移动拥护者可以使用领导力和沟通技巧来教育、培训、协调和促进患者动员 [3, 4, 6],从而帮助发展这种文化。他们以安全和实用技能为重点来支持员工,以提高团队的信心和能力[6]。
结构化的 QI 方法可以极大地促进早期动员和康复的成功实施 [7]。QI 的一种方法包括四个步骤: (1) 总结证据;(2) 识别障碍(例如镇静或缺乏设备);(3) 建立绩效测量(例如,镇静目标、频率和患者活动水平);(4) 确保所有符合条件的患者都接受干预(通过适当的参与、教育、执行和评估)[6, 7]。
一项系统评价确定了早期活动和康复的 28 个独特障碍,包括与患者相关的障碍(例如,生理不稳定性和医疗设备)、结构障碍(例如,有限的人员和设备)、程序障碍(例如,缺乏协调和延迟筛查)资格)和文化障碍(例如,先前的工作人员经验和 ICU 患者护理优先事项)[4]。有许多策略可以有效克服障碍,包括实施安全指南;使用移动协议;跨专业培训、教育和巡查;并包括医生冠军 [4]。
早期动员和康复计划所需的多专业团队合作取决于最佳沟通。我们建议使用适合各个 ICU 的结构来促进跨专业交流,该结构允许(基于算法的)动员目标,包括让所有团队成员有机会提出疑虑并确保有关人员流动目标和成就的信息在员工之间和随着时间的推移流动[8]。
ICU 患者早期活动和康复的经验是可变的。它可能很累、不舒服和困难,而在其他时候对患者来说是一种激励和奖励[9]。随着认知状态的改善,患者可能会对肌肉无力的严重程度感到震惊。在危重疾病的早期阶段,患者可能更愿意专注于多学科团队设定的短期目标(例如,坐在椅子上)[9]。随着患者的病情进展,他们可能会更多地参与目标设定和长期康复计划(例如,走更远的距离,坐在外面)(图 1)。

优化ICU早期活动和康复的十项策略
全尺寸图片荟萃分析已经证明了床内和床外 ICU 活动的安全性,很少发生严重事件 [10]。一种评估安全性的方法是交通灯系统,该系统提供跨呼吸、血液动力学、神经系统和其他身体系统的特定标准,在动员个体患者时要考虑[11]。在该系统中,“红灯”标准表示在动员过程中发生严重安全事件的可能性增加,需要有经验的决策,“黄灯”表示应根据收益与风险进行评估的潜在风险,“绿灯”表示动员通常是安全的 [11]。
患者的镇静和谵妄状态是早期活动和康复的常见障碍[4]。更广泛地说,正如临床指南中所考虑的那样,疼痛、镇静、谵妄、睡眠以及早期活动和康复是密切相关的[3]。通过现有的循证实践(如指南中的综合)对这些问题进行评估和管理,对于最大限度地提高患者参与康复的能力非常重要。
早期活动和康复的障碍可能包括 ICUAW、身体机能受损、外伤和肥胖 [6]。设备可以扩大治疗选择,增加患者的活动能力和活动水平,并降低员工受伤的风险 [12]。选择康复设备可能具有挑战性,重要的考虑因素包括设备成本/可用性、在单位或患者之间共享设备的能力(包括感染控制方面的考虑),以及可供患者活动和方便存放设备的物理空间。支持使用特定设备的证据仍在不断发展,包括评估神经肌肉电刺激 (NMES)、床上自行车测力仪、倾斜台和其他设备 [12, 13]。
在运动方面存在重要的知识差距,包括干预的时间、类型和剂量。有证据表明,在入住 ICU 后 2 或 3 天内开始康复可能优于稍后开始康复 [3]。需要考虑的干预类型包括主动功能动员、床上自行车测功、肌肉电刺激(有或没有被动/主动锻炼)、倾斜台和使用各种康复设备。此外,每种干预类型的强度、持续时间和频率也是重要的考虑因素 [14]。需要进一步研究以进一步了解潜在的益处或危害。在此之前,临床医生的判断将发挥重要作用,并且必须针对个体患者和危重疾病的动态性质进行调整。
需要适合 ICU 环境并融入临床护理的活动和康复相关措施来设定患者目标并跟踪他们的进展,将稀缺的康复资源分配给可能受益最大的患者,并对结构化的质量改进计划进行评估[15]。在患重病之前了解患者的功能以及他们自己的目标,也是重要的考虑因素。
通过正在进行的大型多中心试验,关于 ICU 早期活动的证据仍在不断发展。需要进一步研究以了解干预的最佳时机、类型和剂量,以及它们对长期患者结果的影响。这 10 条策略为在 ICU 中实施早期活动和康复提供指导,目标是优化安全性和有效性,以改善患者的体验和结果。
不适用。
- 1.
Waldauf P、Jiroutkova K、Krajcova A、Puthucheary Z、Duska F。康复干预对危重患者临床结果的影响:随机对照试验的系统评价和荟萃分析。暴击护理医学。2020;48(7):1055–65。
考研谷歌学者
- 2.
小费 CJ、Harrold M、Holland A、Romero L、Nisbet T、Hodgson CL。ICU 积极活动和康复对死亡率和功能的影响:系统评价。重症监护医学。2017;43(2):171–83。
文章 谷歌学术
- 3.
Lang JK、Paykel MS、Haines KJ、Hodgson CL。ICU 早期活动的临床实践指南:系统评价。暴击护理医学。2020;48(11):e1121-8。
文章 谷歌学术
- 4.
Dubb R、Nydahl P、Hermes C、Schwabbauer N、Toonstra A、Parker AM、Kaltwasser A、Needham DM。重症监护病房患者早期活动的障碍和策略。Ann Am Thorac Soc。2016;13(5):724–30。
文章 谷歌学术
- 5.
Hodgson C、Bellomo R、Berney S、Bailey M、Buhr H、Denehy L、Harrold M、Higgins A、Presneill J、Saxena M 等。ICU 机械通气患者的早期活动和恢复:一项双边、多中心、前瞻性队列研究。Crit Care(英国伦敦)。2015;19:81。
文章 谷歌学术
- 6.
帕里 SM、尼达尔 P、尼达姆 DM。在 ICU 中实施早期身体康复和活动:机构、临床医生和患者的考虑。重症监护医学。2018 年;44(4):470–3。
文章 谷歌学术
- 7.
Needham DM、Korupolu R. 重症监护病房环境中的康复质量改进:质量改进模型的实施。顶级中风康复。2010;17(4):271–81。
文章 谷歌学术
- 8.
Schaller SJ、Anstey M、Blobner M、Edrich T、Grabitz SD、Gradwohl-Matis I、Heim M、Houle T、Kurth T、Latronico N 等。外科重症监护病房的早期目标导向动员:一项随机对照试验。柳叶刀。2016;388(10052):1377–88。
文章 谷歌学术
- 9.
角落 EJ、默里 EJ、布雷特 SJ。对患者危重病后早期活动、康复和康复体验的定性、扎根理论探索。BMJ 公开赛。2019;9(2):e026348。
文章 谷歌学术
- 10
Nydahl P、Sricharoenchai T、Chandra S、Kundt FS、Huang M、Fischill M、Needham DM。重症监护病房中患者活动和康复的安全性。系统评价与荟萃分析。Ann Am Thorac Soc。2017;14(5):766–77。
文章 谷歌学术
- 11.
Hodgson CL、Stiller K、Needham DM、Tipping CJ、Harrold M、Baldwin CE、Bradley S、Berney S、Caruana LR、Elliott D 等。机械通气危重成人主动活动安全标准的专家共识和建议。暴击护理(英国伦敦)。2014;18(6):658。
文章 谷歌学术
- 12.
Darragh AR、Campo MA、Frost L、Miller M、Pentico M、Margulis H。治疗实践中的安全患者处理设备:对康复的影响。Am J Occup Ther。2013;67(1):45–53。
文章 谷歌学术
- 13.
Zayed Y、Kheiri B、Barbarawi M、Chahine A、Rashdan L、Chintalapati S、Bachuwa G、Al-Sanouri I。神经肌肉电刺激对危重患者的影响:随机对照试验的系统评价和荟萃分析。Aust 暴击护理。2020;33(2):203-10。
CAS 文章 Google Scholar
- 14.
帕顿 M,车道 R,保罗 E,卡斯伯森 GA,霍奇森 CL。危重病期间的动员:更高水平的动员可改善 6 个月时的健康状况,这是一项前瞻性队列研究的二次分析。暴击护理医学。2021;49(9):e860-9。https://doi.org/10.1097/CCM.0000000000005058。
CAS 文章 PubMed Google Scholar
- 15.
Parry SM、Remedios L、Denehy L、Knight LD、Beach L、Rollinson TC、Berney S、Puthucheary ZA、Morris P、Granger CL。哪些因素会影响早期康复在重症监护病房实践中的实施?与临床医生的定性研究。J 暴击护理。2017;38:137-43。
文章 谷歌学术
下载参考
没有任何。
Carol Hodgson 教授得到了国家健康和医学研究委员会调查员赠款的支持。
隶属关系
澳大利亚和新西兰重症监护研究中心,公共卫生和预防医学学院,莫纳什大学,3/553 St Kilda Rd, Melbourne, VIC, 3004, Australia
卡罗尔·L·霍奇森
澳大利亚维多利亚州墨尔本阿尔弗雷德重症监护和高压医学部
卡罗尔·L·霍奇森
Charité – Universitätsmedizin Berlin,德国柏林自由大学法人会员
斯蒂芬·J·夏勒
麻醉学和手术重症监护医学系,柏林洪堡大学,Chariteplatz 1,柏林,德国
斯蒂芬·J·夏勒
德国慕尼黑工业大学医学院麻醉学和重症监护系
斯蒂芬·J·夏勒
德国基尔石勒苏益格-荷尔斯泰因大学医院麻醉学和重症监护医学系护理研究
彼得·尼达尔
重症监护科,医院以色列阿尔伯特爱因斯坦,圣保罗,SP,巴西
卡琳娜·塔瓦雷斯·蒂涅茨基
美国马里兰州巴尔的摩约翰霍普金斯大学医学院物理医学和康复系肺病和重症监护医学科 (OACIS) 小组
戴尔·M·尼达姆
美国马里兰州巴尔的摩约翰霍普金斯大学护理学院
戴尔·M·尼达姆
- Carol L. Hodgson查看作者出版物
您也可以在PubMed Google Scholar中搜索此作者
- Stefan J. Schaller查看作者出版物
您也可以在PubMed Google Scholar中搜索此作者
- Peter Nydahl查看作者出版物
您也可以在PubMed Google Scholar中搜索此作者
- Karina Tavares Timenetsky查看作者出版物
您也可以在PubMed Google Scholar中搜索此作者
- Dale M. Needham查看作者出版物
您也可以在PubMed Google Scholar中搜索此作者
贡献
CLH 和 DMN 构思了手稿,所有作者都参与了手稿的准备、编辑和最终审查。
通讯作者
与 Carol L. Hodgson 的通信。
伦理批准和同意参与
不适用。
同意发表
不适用。
利益冲突
CH 是 NHMRC 资助的多中心 III 期随机试验的首席研究员,该试验评估重症监护中的早期动员 (GNT1120319)。DMN 是 NIH 资助的多中心随机试验 (R01HL132887) 的首席研究员,该试验评估了急性呼吸衰竭中的营养和运动。该试验已获得 Baxter Healthcare Corporation 的无限制研究资助和捐赠的氨基酸产品,以及 Reck Medical Devices 的设备贷款。DMN 之前曾为 Haisco-USA Pharmaceuticals、Novartis Pharma(瑞士)和 GlaxoSmithKline(英国)提供咨询。对于所有其他作者,不存在与本手稿相关的利益冲突。
出版商说明
Springer Nature 对已发布地图和机构附属机构中的管辖权主张保持中立。
开放获取本文根据知识共享署名 4.0 国际许可协议获得许可,允许以任何媒体或格式使用、共享、改编、分发和复制,只要您适当注明原作者和来源,提供链接到知识共享许可,并指出是否进行了更改。本文中的图像或其他第三方材料包含在文章的知识共享许可中,除非在材料的信用额度中另有说明。如果文章的知识共享许可中未包含材料,并且您的预期用途未得到法律法规的允许或超出允许的用途,则您需要直接从版权所有者处获得许可。要查看此许可证的副本,请访问 http://creativecommons.org/licenses/by/4.0/。
重印和许可
引用这篇文章
Hodgson, CL, Schaller, SJ, Nydahl, P.等。优化重症监护早期活动和康复的十项策略。暴击护理 25, 324 (2021)。https://doi.org/10.1186/s13054-021-03741-z
下载引文
收到:
接受:
发表:
DOI : https://doi.org/10.1186/s13054-021-03741-z











































 京公网安备 11010802027423号
京公网安备 11010802027423号