Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2021-09-03 , DOI: 10.1016/j.apcatb.2021.120685 Yue Yin 1 , Ruolin Lv 1 , Xiaoyang Li 1 , Lu Lv 1, 2, 3 , Weiming Zhang 1, 2, 3
|
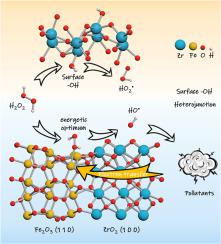
Bimetallic Fenton catalyst has attracted widespread attention in refractory organics removal. Herein, we firstly investigated the influence of ZrO2 structure features on H2O2 activation in Zr-Fe bimetallic catalyst. The results show that the heterojunction structure will be formed after high temperature calcination, which makes the ability of Zr-Fe bimetallic catalyst to activate H2O2 for bisphenol A degradation is 3.1 times higher than that of α-Fe2O3 without Zr doping. Through characterization and density functional theory, it was identified that compared to (1 1 0) interface, the adsorption energy of H2O2 on iron sites at (1 1 0)-(1 0 0) interface reduced by 1.27 kJ mol−1, while the Fe–O bond length in the stable configuration of Fe-OOH increased by 0.16 Å, which was beneficial to the association and dissociation of H2O2 on iron sites. Besides, the surface -OH of amorphous ZrO2 in Zr-Fe bimetallic catalyst synthesized under low temperature conditions could promote HO2•/O2•- formation through the surface electron transfer, accelerating the  Fe(III) reduction. In conclusion, this study reveals the influence of environment-friendly ZrO2 structure features on H2O2 activation, proposes a new insight into strengthening the synergistic effect between bimetals, and provides a reference for the structural optimization of bimetallic catalysts.
Fe(III) reduction. In conclusion, this study reveals the influence of environment-friendly ZrO2 structure features on H2O2 activation, proposes a new insight into strengthening the synergistic effect between bimetals, and provides a reference for the structural optimization of bimetallic catalysts.
中文翻译:

探索 ZrO2 结构特征对 Zr-Fe 双金属催化剂中 H2O2 活化的机制
双金属芬顿催化剂在难降解有机物去除方面受到广泛关注。在此,我们首先研究了 ZrO 2结构特征对Zr-Fe 双金属催化剂中H 2 O 2活化的影响。结果表明,高温煅烧后会形成异质结结构,这使得Zr-Fe双金属催化剂活化H 2 O 2降解双酚A的能力是不含Zr的α-Fe 2 O 3 的3.1倍兴奋剂。通过表征和密度泛函理论,确定与(1 1 0)界面相比,H 2 O 2的吸附能(1 1 0)-(1 0 0) 界面铁位点减少 1.27 kJ mol -1,而稳定构型 Fe-OOH 中的 Fe-O 键长增加 0.16 Å,有利于缔合和 H 2 O 2在铁位点上的解离。此外,低温条件下合成的Zr-Fe双金属催化剂中无定形ZrO 2的表面-OH可以通过表面电子转移促进HO 2 • /O 2 •-的形成,加速 Fe(III)的还原。总之,本研究揭示了环境友好型 ZrO 2结构特征对 H 2 O 2 的影响 活化,提出了加强双金属间协同效应的新见解,并为双金属催化剂的结构优化提供了参考。
Fe(III)的还原。总之,本研究揭示了环境友好型 ZrO 2结构特征对 H 2 O 2 的影响 活化,提出了加强双金属间协同效应的新见解,并为双金属催化剂的结构优化提供了参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号