当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Biochemical Studies Enlighten the Unspecific Peroxygenase from Hypoxylon sp. EC38 as an Efficient Oxidative Biocatalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acscatal.1c03065 Laura Rotilio 1 , Alexander Swoboda 2 , Katharina Ebner 3 , Claudia Rinnofner 3 , Anton Glieder 3 , Wolfgang Kroutil 2, 4, 5 , Andrea Mattevi 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acscatal.1c03065 Laura Rotilio 1 , Alexander Swoboda 2 , Katharina Ebner 3 , Claudia Rinnofner 3 , Anton Glieder 3 , Wolfgang Kroutil 2, 4, 5 , Andrea Mattevi 1
Affiliation
|
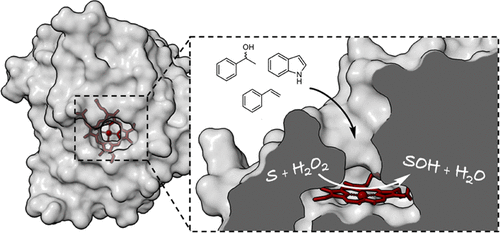
Unspecific peroxygenases (UPOs) are glycosylated fungal enzymes that can selectively oxidize C–H bonds. UPOs employ hydrogen peroxide as the oxygen donor and reductant. With such an easy-to-handle cosubstrate and without the need for a reducing agent, UPOs are emerging as convenient oxidative biocatalysts. Here, an unspecific peroxygenase from Hypoxylon sp. EC38 (HspUPO) was identified in an activity-based screen of six putative peroxygenase enzymes that were heterologously expressed in Pichia pastoris. The enzyme was found to tolerate selected organic solvents such as acetonitrile and acetone. HspUPO is a versatile catalyst performing various reactions, such as the oxidation of prim- and sec-alcohols, epoxidations, and hydroxylations. Semipreparative biotransformations were demonstrated for the nonenantioselective oxidation of racemic 1-phenylethanol rac-1b (TON = 13 000), giving the product with 88% isolated yield, and the oxidation of indole 6a to give indigo 6b (TON = 2800) with 98% isolated yield. HspUPO features a compact and rigid three-dimensional conformation that wraps around the heme and defines a funnel-shaped tunnel that leads to the heme iron from the protein surface. The tunnel extends along a distance of about 12 Å with a fairly constant diameter in its innermost segment. Its surface comprises both hydrophobic and hydrophilic groups for dealing with substrates of variable polarities. The structural investigation of several protein–ligand complexes revealed that the active site of HspUPO is accessible to molecules of varying bulkiness with minimal or no conformational changes, explaining the relatively broad substrate scope of the enzyme. With its convenient expression system, robust operational properties, relatively small size, well-defined structural features, and diverse reaction scope, HspUPO is an exploitable candidate for peroxygenase-based biocatalysis.
中文翻译:

结构和生化研究揭示了来自 Hypoxylon sp. 的非特异性过氧化酶。EC38 作为一种高效的氧化生物催化剂
非特异性过氧化酶 (UPO) 是糖基化的真菌酶,可以选择性氧化 C-H 键。UPO 使用过氧化氢作为氧供体和还原剂。凭借这种易于处理的共底物且无需还原剂,UPO 正成为一种方便的氧化生物催化剂。在这里,来自Hypoxylon sp.的非特异性过氧化酶。EC 38 ( Hsp UPO) 在六种假定的过氧化酶的基于活性的筛选中被鉴定出来,这些酶在毕赤酵母中异源表达。发现该酶耐受选定的有机溶剂,例如乙腈和丙酮。Hsp UPO 是一种多功能催化剂,可进行各种反应,例如原液的氧化- 和仲醇、环氧化和羟基化。外消旋 1-苯基乙醇rac - 1b (TON = 13 000) 的非对映选择性氧化证明了半制备性生物转化,产物的分离产率为 88%,吲哚6a氧化为靛蓝6b (TON = 2800),分离产率为98%分离产量。热敏电阻UPO 具有紧凑而刚性的三维构象,它包裹着血红素,并定义了一个漏斗形隧道,从蛋白质表面通向血红素铁。隧道沿着约 12 埃的距离延伸,其最内段的直径相当恒定。其表面包含疏水基团和亲水基团,用于处理不同极性的基材。几种蛋白质 - 配体复合物的结构研究表明,Hsp UPO的活性位点可用于不同体积的分子,而构象变化很小或没有,这解释了该酶相对广泛的底物范围。凭借其便捷的表达系统、强大的操作性能、相对较小的尺寸、明确的结构特征和多样化的反应范围,Hsp UPO 是基于过氧化酶的生物催化的可利用候选者。
更新日期:2021-09-17
中文翻译:

结构和生化研究揭示了来自 Hypoxylon sp. 的非特异性过氧化酶。EC38 作为一种高效的氧化生物催化剂
非特异性过氧化酶 (UPO) 是糖基化的真菌酶,可以选择性氧化 C-H 键。UPO 使用过氧化氢作为氧供体和还原剂。凭借这种易于处理的共底物且无需还原剂,UPO 正成为一种方便的氧化生物催化剂。在这里,来自Hypoxylon sp.的非特异性过氧化酶。EC 38 ( Hsp UPO) 在六种假定的过氧化酶的基于活性的筛选中被鉴定出来,这些酶在毕赤酵母中异源表达。发现该酶耐受选定的有机溶剂,例如乙腈和丙酮。Hsp UPO 是一种多功能催化剂,可进行各种反应,例如原液的氧化- 和仲醇、环氧化和羟基化。外消旋 1-苯基乙醇rac - 1b (TON = 13 000) 的非对映选择性氧化证明了半制备性生物转化,产物的分离产率为 88%,吲哚6a氧化为靛蓝6b (TON = 2800),分离产率为98%分离产量。热敏电阻UPO 具有紧凑而刚性的三维构象,它包裹着血红素,并定义了一个漏斗形隧道,从蛋白质表面通向血红素铁。隧道沿着约 12 埃的距离延伸,其最内段的直径相当恒定。其表面包含疏水基团和亲水基团,用于处理不同极性的基材。几种蛋白质 - 配体复合物的结构研究表明,Hsp UPO的活性位点可用于不同体积的分子,而构象变化很小或没有,这解释了该酶相对广泛的底物范围。凭借其便捷的表达系统、强大的操作性能、相对较小的尺寸、明确的结构特征和多样化的反应范围,Hsp UPO 是基于过氧化酶的生物催化的可利用候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号