当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural and functional analysis of the C-terminal region of Streptococcus gordonii SspB
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-09-02 , DOI: 10.1107/s2059798321008135 Norbert Schormann 1 , Sangeetha Purushotham 1 , Joshua L Mieher 1 , Manisha Patel 1 , Hui Wu 2 , Champion Deivanayagam 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-09-02 , DOI: 10.1107/s2059798321008135 Norbert Schormann 1 , Sangeetha Purushotham 1 , Joshua L Mieher 1 , Manisha Patel 1 , Hui Wu 2 , Champion Deivanayagam 1
Affiliation
|
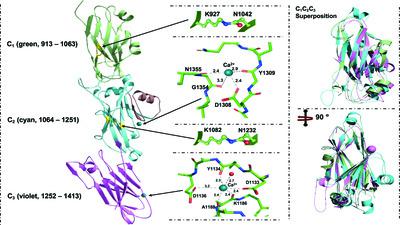
Streptococcus gordonii is a member of the viridans streptococci and is an early colonizer of the tooth surface. Adherence to the tooth surface is enabled by proteins present on the S. gordonii cell surface, among which SspB belongs to one of the most well studied cell-wall-anchored adhesin families: the antigen I/II (AgI/II) family. The C-terminal region of SspB consists of three tandemly connected individual domains that display the DEv-IgG fold. These C-terminal domains contain a conserved Ca2+-binding site and isopeptide bonds, and they adhere to glycoprotein 340 (Gp340; also known as salivary agglutinin, SAG). Here, the structural and functional characterization of the C123SspB domain at 2.7 Å resolution is reported. Although the individual C-terminal domains of Streptococcus mutans AgI/II and S. gordonii SspB show a high degree of both sequence and structural homology, superposition of these structures highlights substantial differences in their electrostatic surface plots, and this can be attributed to the relative orientation of the individual domains (C1, C2 and C3) with respect to each other and could reflect their specificity in binding to extracellular matrix molecules. Studies further confirmed that affinity for Gp340 or its scavenger receptor cysteine-rich (SRCR) domains requires two of the three domains of C123SspB, namely C12 or C23, which is different from AgI/II. Using protein–protein docking studies, models for this observed functional difference between C123SspB and C123AgI/II in their binding to SRCR1 are presented.
中文翻译:

戈登链球菌 SspB C 末端区域的结构和功能分析
戈登链球菌是草绿色链球菌的一员,是牙齿表面的早期定殖者。牙齿表面的粘附是通过存在于S. gordonii细胞表面的蛋白质实现的,其中 SspB 属于研究最深入的细胞壁锚定粘附素家族之一:抗原 I/II (AgI/II) 家族。SspB 的 C 端区域由三个串联连接的单独结构域组成,显示 DEv-IgG 折叠。这些C末端结构域含有保守的Ca 2+结合位点和异肽键,并且它们粘附至糖蛋白340(Gp340;也称为唾液凝集素,SAG)。在此,报告了 2.7 Å 分辨率下C 123 SspB结构域的结构和功能表征。尽管变形链球菌AgI/II 和戈氏链球菌SspB的各个 C 端结构域显示出高度的序列和结构同源性,但这些结构的叠加突出了它们静电表面图的显着差异,这可归因于相对各个结构域(C 1、C 2和C 3)相对于彼此的方向,并且可以反映它们与细胞外基质分子结合的特异性。研究进一步证实,对Gp340或其清道夫受体富含半胱氨酸(SRCR)结构域的亲和力需要C 123 SspB的三个结构域中的两个,即C 12或C 23,这与AgI/II不同。使用蛋白质-蛋白质对接研究,提出了C 123 SspB和 C 123 AgI/II在与 SRCR 1结合方面观察到的功能差异的模型。
更新日期:2021-09-02
中文翻译:

戈登链球菌 SspB C 末端区域的结构和功能分析
戈登链球菌是草绿色链球菌的一员,是牙齿表面的早期定殖者。牙齿表面的粘附是通过存在于S. gordonii细胞表面的蛋白质实现的,其中 SspB 属于研究最深入的细胞壁锚定粘附素家族之一:抗原 I/II (AgI/II) 家族。SspB 的 C 端区域由三个串联连接的单独结构域组成,显示 DEv-IgG 折叠。这些C末端结构域含有保守的Ca 2+结合位点和异肽键,并且它们粘附至糖蛋白340(Gp340;也称为唾液凝集素,SAG)。在此,报告了 2.7 Å 分辨率下C 123 SspB结构域的结构和功能表征。尽管变形链球菌AgI/II 和戈氏链球菌SspB的各个 C 端结构域显示出高度的序列和结构同源性,但这些结构的叠加突出了它们静电表面图的显着差异,这可归因于相对各个结构域(C 1、C 2和C 3)相对于彼此的方向,并且可以反映它们与细胞外基质分子结合的特异性。研究进一步证实,对Gp340或其清道夫受体富含半胱氨酸(SRCR)结构域的亲和力需要C 123 SspB的三个结构域中的两个,即C 12或C 23,这与AgI/II不同。使用蛋白质-蛋白质对接研究,提出了C 123 SspB和 C 123 AgI/II在与 SRCR 1结合方面观察到的功能差异的模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号