当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Access to Amides from Nitro-Compounds via Aminocarbonylation and Amidation Reactions: A Minireview
The Chemical Record ( IF 7.0 ) Pub Date : 2021-09-02 , DOI: 10.1002/tcr.202100224 Dinesh S Barak 1 , Sanjay Batra 1, 2
The Chemical Record ( IF 7.0 ) Pub Date : 2021-09-02 , DOI: 10.1002/tcr.202100224 Dinesh S Barak 1 , Sanjay Batra 1, 2
Affiliation
|
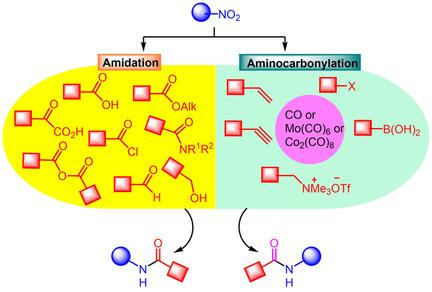
The ubiquity of the amide bond in functional molecules including proteins, natural products, pharmaceuticals, agrochemicals and materials provides impetus to design and develop newer strategies for the generation of this linkage. Owing to growing awareness about sustainability and development of benign strategies, the traditional route of synthesis of amides via reaction between carboxylic acids and amines in the presence of stoichiometric amount of coupling reagents is tagged to be harsh and wasteful. In one of the unconventional routes, nitro compounds are used directly as amine surrogates for preparing amides mostly via aminocarbonylation and amidation reactions. Typically, such processes involves nitroarenes owing to their propensity to transform into nitroso, hydroxylamine, diazo, hydrazine or aniline intermediates in situ under the influence of suitable catalyst or oxidant. This short review provides the comprehensive overview of these reactions including insight into the scope and their mechanisms.
中文翻译:

通过氨基羰基化和酰胺化反应直接从硝基化合物中获得酰胺:一个小综述
酰胺键在功能分子(包括蛋白质、天然产物、药物、农用化学品和材料)中的普遍存在为设计和开发产生这种键的更新策略提供了动力。由于对可持续性和良性策略发展的认识不断提高,在化学计量的偶联剂存在下,通过羧酸和胺之间的反应合成酰胺的传统途径被标记为苛刻和浪费。在一种非常规路线中,硝基化合物直接用作胺替代物,主要通过氨基羰基化和酰胺化反应制备酰胺。通常,此类过程涉及硝基芳烃,因为它们倾向于转化为亚硝基、羟胺、重氮、在合适的催化剂或氧化剂的影响下原位生成肼或苯胺中间体。这篇简短的评论提供了这些反应的全面概述,包括对范围及其机制的深入了解。
更新日期:2021-09-02
中文翻译:

通过氨基羰基化和酰胺化反应直接从硝基化合物中获得酰胺:一个小综述
酰胺键在功能分子(包括蛋白质、天然产物、药物、农用化学品和材料)中的普遍存在为设计和开发产生这种键的更新策略提供了动力。由于对可持续性和良性策略发展的认识不断提高,在化学计量的偶联剂存在下,通过羧酸和胺之间的反应合成酰胺的传统途径被标记为苛刻和浪费。在一种非常规路线中,硝基化合物直接用作胺替代物,主要通过氨基羰基化和酰胺化反应制备酰胺。通常,此类过程涉及硝基芳烃,因为它们倾向于转化为亚硝基、羟胺、重氮、在合适的催化剂或氧化剂的影响下原位生成肼或苯胺中间体。这篇简短的评论提供了这些反应的全面概述,包括对范围及其机制的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号