Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2021-09-02 , DOI: 10.1016/j.addr.2021.113961 Dhanu Gupta 1 , Antje Maria Zickler 1 , Samir El Andaloussi 1
|
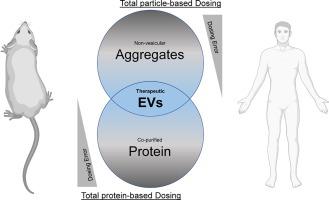
Extracellular vesicles (EVs) are natural nanoparticles containing biologically active molecules. They are important mediators of intercellular communication and can be exploited therapeutically by various bioengineering approaches. To accurately determine the therapeutic potential of EVs in pre-clinical and clinical settings, dependable dosing strategies are of utmost importance. However, the field suffers from inconsistencies comprising all areas of EV production and characterisation. Therefore, a standardised and well-defined process in EV quantification, key to reliable therapeutic EV dosing, remains to be established. Here, we examined 64 pre-clinical studies for EV-based therapeutics with respect to their applied EV dosing strategies. We identified variations in effective dosing strategies irrespective of the applied EV purification method and cell source. Moreover, we found dose discrepancies depending on the disease model, where EV doses were selected without accounting for published EV pharmacokinetics or biodistribution patterns. We therefore propose to focus on qualitative aspects when dosing EV-based therapeutics, such as the potency of the therapeutic cargo entity. This will ensure batch-to-batch reliability and enhance reproducibility between applications. Furthermore, it will allow for the successful benchmarking of EV-based therapeutics compared to other nanoparticle drug delivery systems, such as viral vector-based or lipid-based nanoparticle approaches.
中文翻译:

细胞外囊泡给药
细胞外囊泡 (EV) 是含有生物活性分子的天然纳米颗粒。它们是细胞间通讯的重要介质,可以通过各种生物工程方法在治疗上加以利用。为了准确确定 EV 在临床前和临床环境中的治疗潜力,可靠的给药策略至关重要。然而,该领域在电动汽车生产和表征的所有领域都存在不一致的问题。因此,仍有待建立一个标准化和明确定义的 EV 量化过程,这是可靠治疗性 EV 剂量的关键。在这里,我们检查了 64 项基于 EV 的疗法的临床前研究,以及它们应用的 EV 给药策略。无论应用的 EV 纯化方法和细胞来源如何,我们都确定了有效剂量策略的变化。此外,我们发现剂量差异取决于疾病模型,其中选择 EV 剂量时未考虑已发表的 EV 药代动力学或生物分布模式。因此,我们建议在给予基于 EV 的疗法时关注定性方面,例如治疗货物实体的效力。这将确保批次间的可靠性并提高应用程序之间的可重复性。此外,与其他纳米颗粒药物递送系统(例如基于病毒载体或基于脂质的纳米颗粒方法)相比,它将允许对基于 EV 的疗法进行成功的基准测试。其中 EV 剂量的选择没有考虑已发表的 EV 药代动力学或生物分布模式。因此,我们建议在给予基于 EV 的疗法时关注定性方面,例如治疗货物实体的效力。这将确保批次间的可靠性并提高应用程序之间的可重复性。此外,与其他纳米颗粒药物递送系统(例如基于病毒载体或基于脂质的纳米颗粒方法)相比,它将允许对基于 EV 的疗法进行成功的基准测试。其中 EV 剂量的选择没有考虑已发表的 EV 药代动力学或生物分布模式。因此,我们建议在给予基于 EV 的疗法时关注定性方面,例如治疗货物实体的效力。这将确保批次间的可靠性并提高应用程序之间的可重复性。此外,与其他纳米颗粒药物递送系统(例如基于病毒载体或基于脂质的纳米颗粒方法)相比,它将允许对基于 EV 的疗法进行成功的基准测试。这将确保批次间的可靠性并提高应用程序之间的可重复性。此外,与其他纳米颗粒药物递送系统(例如基于病毒载体或基于脂质的纳米颗粒方法)相比,它将允许对基于 EV 的疗法进行成功的基准测试。这将确保批次间的可靠性并提高应用程序之间的可重复性。此外,与其他纳米颗粒药物递送系统(例如基于病毒载体或基于脂质的纳米颗粒方法)相比,它将允许对基于 EV 的疗法进行成功的基准测试。











































 京公网安备 11010802027423号
京公网安备 11010802027423号