Kidney International ( IF 14.8 ) Pub Date : 2021-09-02 , DOI: 10.1016/j.kint.2021.08.015 Bernd Hoppe 1 , Annelize Koch 2 , Pierre Cochat 3 , Sander F Garrelfs 4 , Michelle A Baum 5 , Jaap W Groothoff 4 , Graham Lipkin 6 , Martin Coenen 7 , Gesa Schalk 7 , Aniruddha Amrite 8 , David McDougall 9 , Kelly Barrios 8 , Craig B Langman 10
|
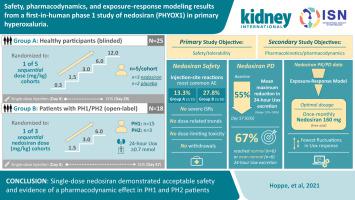
Primary hyperoxaluria (PH) is a family of ultra-rare autosomal recessive inherited disorders of hepatic glyoxylate metabolism characterized by oxalate overproduction. Nedosiran is an RNA interference agent that inhibits hepatic lactate dehydrogenase, the enzyme responsible for the common, final step of oxalate production in all three genetic subtypes of PH. Here, we assessed in a two-part, randomized, single-ascending-dose, phase 1 study (PHYOX1) the safety, pharmacokinetics, pharmacodynamics, and exposure-response of subcutaneous nedosiran in 25 healthy participants (Group A) and 18 patients with PH1 or PH2 (Group B). Group A received nedosiran (0.3, 1.5, 3.0, 6.0, then 12.0 mg/kg) or placebo, and Group B received open-label nedosiran (1.5, 3.0, or 6.0 mg/kg). No significant safety concerns were identified. Injection site reactions (four or more hours post dose) occurred in 13.3% of participants in Group A and 27.8% of participants in Group B. Mean maximum reduction in 24-hour urinary oxalate excretion from baseline to day 57 (end of study) across Group B dose cohorts was 55% (range: 22%–100%) after single-dose nedosiran, with 33% participants reaching normal 24-hour urinary oxalate excretion. Based on the available modeling and simulation data, a fixed monthly dose of nedosiran 160 mg (free acid; equivalent to 170 mg sodium salt) in adults was associated with the highest proportion of simulated individuals achieving normal or near-normal 24-hour urinary oxalate excretion and fewest fluctuations in urinary oxalate response. Thus, single-dose nedosiran demonstrated acceptable safety and evidence of a pharmacodynamic effect in both PH1 and PH2 subpopulations consistent with its mechanism of action.
中文翻译:

nedosiran (PHYOX1) 在原发性高草酸尿症中的首次人体 1 期研究的安全性、药效学和暴露反应建模结果
原发性高草酸尿症 (PH) 是一组超罕见的常染色体隐性遗传的肝脏乙醛酸代谢疾病,其特征是草酸过量产生。Nedosiran 是一种 RNA 干扰剂,可抑制肝乳酸脱氢酶,该酶负责所有三种遗传性 PH 亚型中草酸产生的常见最后一步。在这里,我们在一项由两部分组成的随机单次递增剂量 1 期研究 (PHYOX1) 在 25 名健康参与者(A 组)和 18 名患有PH1 或 PH2(B 组)。A 组接受 nedosiran(0.3、1.5、3.0、6.0,然后 12.0 mg/kg)或安慰剂,B 组接受开放标签 nedosiran(1.5、3.0 或 6.0 mg/kg)。没有发现重大的安全问题。A 组 13.3% 的参与者和 B 组 27.8% 的参与者发生注射部位反应(给药后 4 小时或更长时间)。从基线到第 57 天(研究结束),24 小时尿草酸盐排泄的平均最大减少单剂量 nedosiran 后 B 组剂量组为 55%(范围:22%–100%),33% 的参与者达到正常的 24 小时尿草酸盐排泄。根据可用的建模和模拟数据,成人每月固定剂量的 nedosiran 160 mg(游离酸;相当于 170 mg 钠盐)与达到正常或接近正常 24 小时尿草酸盐的模拟个体比例最高排泄和尿草酸盐反应波动最小。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号