International Journal of Pharmaceutics ( IF 5.3 ) Pub Date : 2021-09-02 , DOI: 10.1016/j.ijpharm.2021.121073 Harshvardhan Modh 1 , Daniel Juncheng Fang 1 , Yi Hsuan Ou 1 , Jia Ning Nicolette Yau 1 , Tatyana Kovshova 2 , Shakti Nagpal 1 , Julian Knoll 3 , Chantal M Wallenwein 4 , Kuntal Maiti 5 , Subhas Bhowmick 5 , Svetlana Gelperina 6 , Giorgia Pastorin 1 , Matthias G Wacker 1
|
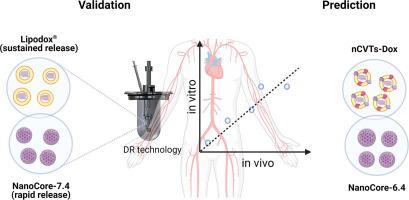
A growing number of nanomedicines entered the clinical trials and improved our understanding of the in vivo responses expected in humans. The in vitro drug release represents an important critical quality attribute involved in pharmacokinetics. Establishing in vitro-in vivo relationships for nanomedicines requires a careful analysis of the clinical data with respect to the unique differences between drugs and nanomedicines. Also, the biorelevant assay must reflect the release mechanism of the carrier. Four drug delivery systems of doxorubicin were evaluated for their in vitro release behavior under biorelevant conditions using the dispersion releaser. The pharmacokinetics observed during the first-in-men clinical trials were analyzed using a custom-made physiologically-based nanocarrier biopharmaceutics model. The drug product Lipodox® and the clinical candidate NanoCore-7.4 were evaluated to validate the model. Afterward, the in vivo performances of the preclinical candidates NanoCore-6.4 and doxorubicin-loaded nano-cellular vesicle technology systems (an extracellular vesicle preparation) were predicted. In vitro and in vivo release were in good correlation as indicated by the coefficients of determination of 0.98648 (NanoCore-7.4) and 0.94107 (Lipodox®). The predictions required an estimation of the carrier half-life in blood circulation leading to considerable uncertainty. Still, the simulations narrow down the possible scenarios in the clinical evaluation of nanomedicines and provide a valuable addition to animal studies.
中文翻译:

重新审视阿霉素的可注射给药系统:使用人类临床数据的体外-体内关系
越来越多的纳米药物进入临床试验,提高了我们对人体预期体内反应的理解。该体外释药代表参与药物代谢动力学的重要关键质量属性。建立纳米药物的体外-体内关系需要仔细分析临床数据,了解药物和纳米药物之间的独特差异。此外,生物相关测定必须反映载体的释放机制。对阿霉素的四种药物递送系统进行了体外评估使用分散体释放剂在生物相关条件下的释放行为。使用定制的基于生理学的纳米载体生物药剂学模型分析了首次人体临床试验期间观察到的药代动力学。对药物产品 Lipodox® 和临床候选药物 NanoCore-7.4 进行了评估以验证模型。随后,预测了临床前候选药物 NanoCore-6.4 和载有阿霉素的纳米细胞囊泡技术系统(一种细胞外囊泡制剂)的体内性能。体外和体内0.98648 (NanoCore-7.4) 和 0.94107 (Lipodox®) 的测定系数表明,释放具有良好的相关性。预测需要估计血液循环中的载体半衰期,导致相当大的不确定性。尽管如此,模拟缩小了纳米药物临床评估中可能出现的情况,并为动物研究提供了有价值的补充。











































 京公网安备 11010802027423号
京公网安备 11010802027423号