当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective difunctionalization of electron-deficient alkynes: access to (E)-2-iodo-3-(methylthio)acrylate
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-26 , DOI: 10.1039/d1ob01287h Ling-Hui Lu 1 , Guangchuan Ou 1 , Xiongjie Zhao 1 , Yufei Wang 1 , Xuelian Chen 1 , Wenjun Liao 1 , Shundan Li 1 , Chao Wu 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-26 , DOI: 10.1039/d1ob01287h Ling-Hui Lu 1 , Guangchuan Ou 1 , Xiongjie Zhao 1 , Yufei Wang 1 , Xuelian Chen 1 , Wenjun Liao 1 , Shundan Li 1 , Chao Wu 1
Affiliation
|
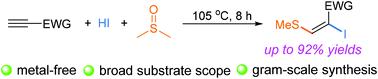
A convenient and efficient approach to (E)-2-iodo-3-(methylthio)acrylate has been developed through direct iodothiomethylation of alkynes with aqueous HI and DMSO under mild conditions. This novel protocol has demonstrated a unique difunctionalization of electron-deficient alkynes with a broad substrate scope and excellent functional-group tolerance. Preliminary mechanistic studies indicated that prior diiodination of alkynes, followed by nucleophilic substitution with in situ generated DMS led to the formation of (E)-2-iodo-3-(methylthio)acrylate.
中文翻译:

缺电子炔烃的选择性双官能化:获得(E)-2-碘-3-(甲硫基)丙烯酸酯
通过在温和条件下用 HI 和 DMSO 水溶液直接碘硫甲基化炔烃,开发了一种方便有效的 ( E )-2-iodo-3-(甲硫基)丙烯酸酯方法。这种新颖的协议证明了缺电子炔烃的独特双功能化,具有广泛的底物范围和出色的官能团耐受性。初步机理研究表明,先对炔烃进行二碘化,然后用原位产生的 DMS 进行亲核取代,导致形成 ( E )-2-iodo-3-(methylthio) 丙烯酸酯。
更新日期:2021-09-02
中文翻译:

缺电子炔烃的选择性双官能化:获得(E)-2-碘-3-(甲硫基)丙烯酸酯
通过在温和条件下用 HI 和 DMSO 水溶液直接碘硫甲基化炔烃,开发了一种方便有效的 ( E )-2-iodo-3-(甲硫基)丙烯酸酯方法。这种新颖的协议证明了缺电子炔烃的独特双功能化,具有广泛的底物范围和出色的官能团耐受性。初步机理研究表明,先对炔烃进行二碘化,然后用原位产生的 DMS 进行亲核取代,导致形成 ( E )-2-iodo-3-(methylthio) 丙烯酸酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号