当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleophile-induced transformation of phenoxathiin-based thiacalixarenes
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-20 , DOI: 10.1039/d1ob01487k Tomáš Landovský 1 , Martin Babor 2 , Jan Čejka 2 , Václav Eigner 2 , Hana Dvořáková 3 , Martin Krupička 1 , Pavel Lhoták 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-20 , DOI: 10.1039/d1ob01487k Tomáš Landovský 1 , Martin Babor 2 , Jan Čejka 2 , Václav Eigner 2 , Hana Dvořáková 3 , Martin Krupička 1 , Pavel Lhoták 1
Affiliation
|
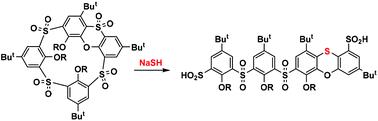
Oxidized phenoxathiin-based macrocycles, easily accessible thiacalix[4]arene derivatives, consist of a unique set of structural elements representing a key prerequisite for the unexpected reactivity described in this paper. As proposed, the internal strain, imposed by the presence of a heterocyclic moiety, together with a number of electron-withdrawing groups (SO2) opens the way to the cleavage of the macrocyclic skeleton through a cascade of three SNAr reactions triggered by the nucleophilic attack of an SH− anion. The whole transformation, which is unparalleled in classical calixarene chemistry, leads to unique linear sulfinic acid derivatives with a rearranged phenoxathiin moiety that can serve as building blocks for macrocyclic systems of a new type.
中文翻译:

亲核试剂诱导的吩氧噻吩基硫杯芳烃转化
基于氧化吩氧噻吩的大环化合物,易于获得的硫杯 [4] 芳烃衍生物,由一组独特的结构元素组成,代表了本文中描述的意外反应性的关键先决条件。正如所提出的,由杂环部分的存在以及许多吸电子基团 (SO 2 )的存在所施加的内部应变为通过三个 S N Ar 反应触发的级联反应打开了大环骨架裂解的途径。 SH -阴离子的亲核攻击。整个转化在经典杯芳烃化学中是无与伦比的,导致独特的线性亚磺酸衍生物具有重排的吩氧噻吩部分,可作为新型大环系统的构建单元。
更新日期:2021-09-02
中文翻译:

亲核试剂诱导的吩氧噻吩基硫杯芳烃转化
基于氧化吩氧噻吩的大环化合物,易于获得的硫杯 [4] 芳烃衍生物,由一组独特的结构元素组成,代表了本文中描述的意外反应性的关键先决条件。正如所提出的,由杂环部分的存在以及许多吸电子基团 (SO 2 )的存在所施加的内部应变为通过三个 S N Ar 反应触发的级联反应打开了大环骨架裂解的途径。 SH -阴离子的亲核攻击。整个转化在经典杯芳烃化学中是无与伦比的,导致独特的线性亚磺酸衍生物具有重排的吩氧噻吩部分,可作为新型大环系统的构建单元。











































 京公网安备 11010802027423号
京公网安备 11010802027423号