当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Associating High Oxide-Ion Conductivity and Conduction Mechanisms with Local Atomic Environments in Na0.5Bi0.5–xTi1–yMgyO3−δ
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.jpcc.1c04995 B. Santhoshkumar 1 , K. R. Priolkar 1 , Simone Pollastri 2 , Danilo Oliveira de Souza 2 , Ilaria Carlomagno 2 , A. K. Bera 3 , S. M. Yusuf 3, 4 , Bholanath Pahari 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.jpcc.1c04995 B. Santhoshkumar 1 , K. R. Priolkar 1 , Simone Pollastri 2 , Danilo Oliveira de Souza 2 , Ilaria Carlomagno 2 , A. K. Bera 3 , S. M. Yusuf 3, 4 , Bholanath Pahari 1
Affiliation
|
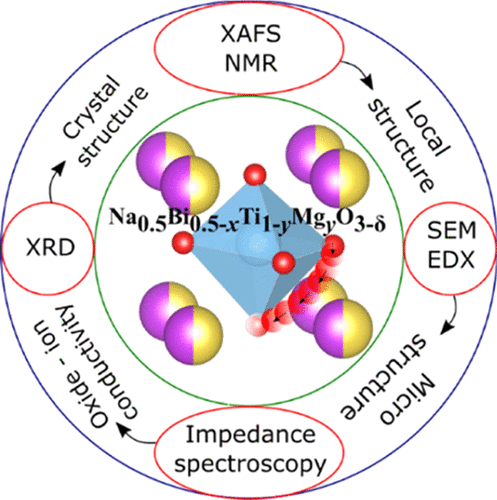
Oxide-ion conductors are of high interest in electrochemical devices such as solid-oxide fuel cells, oxygen sensors, and separation membranes. In this paper, high oxide-ion conductivity and associated ion conduction mechanism in perovskite-type oxides Na0.5Bi0.5–xTi1–yMgyO3–1.5x–y (for x = 0.0 and y = 0.0, x = 0.01 and y = 0.02, x = 0.01 and y = 0.04) are investigated systematically. Na0.5Bi0.5TiO3 ceramic is a poor conductor, whereas Na0.5Bi0.49Ti0.98Mg0.02O2.965 and Na0.5Bi0.49Ti0.96Mg0.04O2.945 ceramics are excellent oxide-ion conductors at 500 °C. While the Rietveld refinements of powder X-ray diffraction data using the monoclinic Cc space group and the rhombohedral R3c space group showed reasonably similar quality of fits, extended X-ray absorption fine structure (EXAFS) data could be fitted only with the monoclinic Cc structure at room temperature for all three ceramics. Extensive EXAFS investigations have also been used to probe the local environments of Bi and Ti atoms directly and reveal the ordering of Bi3+/Na+, displacements of the cations, oxygen-vacancy generation, and their migration pathways. Our EXAFS results demonstrate Bi- and Na-rich planes formation due to short-range ordering of Bi3+/Na+ in the perovskite units. Oxygen vacancies were found to be located in the Bi-rich planes. 23Na magic-angle spinning NMR experiments indicate that the local environments of Na atoms are disordered. The present work also provides an insight into the dramatically improved conducting behavior of Na0.5Bi0.49Ti0.98Mg0.02O2.965 and Na0.5Bi0.49Ti0.96Mg0.04O2.945 ceramics in terms of the local, long-range, and microstructure, which can be exploited to develop design principles for the syntheses of related oxides with even improved properties.
中文翻译:

在 Na0.5Bi0.5–xTi1–yMgyO3−δ 中将高氧化物离子电导率和传导机制与局部原子环境相关联
氧化物离子导体在诸如固体氧化物燃料电池、氧传感器和分离膜之类的电化学装置中具有很高的兴趣。在本文中,钙钛矿型氧化物 Na 0.5 Bi 0.5– x Ti 1– y Mg y O 3–1.5 x – y(对于x = 0.0 和y = 0.0,x = 0.01 和y = 0.02,x = 0.01 和y = 0.04) 被系统地研究。Na 0.5 Bi 0.5 TiO 3陶瓷是不良导体,而 Na 0.5 Bi 0.49 Ti 0.98 Mg 0.02 O 2.965和 Na 0.5 Bi 0.49 Ti 0.96 Mg 0.04 O 2.945陶瓷在 500 °C 下是优良的氧化物离子导体。虽然使用单斜Cc空间群和菱形R 3 c空间群的粉末 X 射线衍射数据的 Rietveld 精修显示出相当相似的拟合质量,但扩展 X 射线吸收精细结构 (EXAFS) 数据只能与单斜抄送所有三种陶瓷在室温下的结构。广泛的 EXAFS 研究也已用于直接探测 Bi 和 Ti 原子的局部环境,并揭示 Bi 3+ /Na +的排序、阳离子的位移、氧空位的产生及其迁移途径。我们的 EXAFS 结果表明,由于钙钛矿单元中 Bi 3+ /Na +的短程排序,形成了富含 Bi 和 Na 的平面。发现氧空位位于富铋平面。23 Na魔角自旋核磁共振实验表明Na原子的局部环境是无序的。目前的工作还提供了对 Na 0.5显着改善的导电行为的见解Bi 0.49 Ti 0.98 Mg 0.02 O 2.965和 Na 0.5 Bi 0.49 Ti 0.96 Mg 0.04 O 2.945陶瓷在局部、长程和微观结构方面,可用于开发相关氧化物合成的设计原则,甚至改进特性。
更新日期:2021-09-16
中文翻译:

在 Na0.5Bi0.5–xTi1–yMgyO3−δ 中将高氧化物离子电导率和传导机制与局部原子环境相关联
氧化物离子导体在诸如固体氧化物燃料电池、氧传感器和分离膜之类的电化学装置中具有很高的兴趣。在本文中,钙钛矿型氧化物 Na 0.5 Bi 0.5– x Ti 1– y Mg y O 3–1.5 x – y(对于x = 0.0 和y = 0.0,x = 0.01 和y = 0.02,x = 0.01 和y = 0.04) 被系统地研究。Na 0.5 Bi 0.5 TiO 3陶瓷是不良导体,而 Na 0.5 Bi 0.49 Ti 0.98 Mg 0.02 O 2.965和 Na 0.5 Bi 0.49 Ti 0.96 Mg 0.04 O 2.945陶瓷在 500 °C 下是优良的氧化物离子导体。虽然使用单斜Cc空间群和菱形R 3 c空间群的粉末 X 射线衍射数据的 Rietveld 精修显示出相当相似的拟合质量,但扩展 X 射线吸收精细结构 (EXAFS) 数据只能与单斜抄送所有三种陶瓷在室温下的结构。广泛的 EXAFS 研究也已用于直接探测 Bi 和 Ti 原子的局部环境,并揭示 Bi 3+ /Na +的排序、阳离子的位移、氧空位的产生及其迁移途径。我们的 EXAFS 结果表明,由于钙钛矿单元中 Bi 3+ /Na +的短程排序,形成了富含 Bi 和 Na 的平面。发现氧空位位于富铋平面。23 Na魔角自旋核磁共振实验表明Na原子的局部环境是无序的。目前的工作还提供了对 Na 0.5显着改善的导电行为的见解Bi 0.49 Ti 0.98 Mg 0.02 O 2.965和 Na 0.5 Bi 0.49 Ti 0.96 Mg 0.04 O 2.945陶瓷在局部、长程和微观结构方面,可用于开发相关氧化物合成的设计原则,甚至改进特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号