当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereomers and Low-Temperature Oxidation
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.jpca.1c05635 Aaron D Danilack 1 , Clayton R Mulvihill 2 , Stephen J Klippenstein 2 , C Franklin Goldsmith 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.jpca.1c05635 Aaron D Danilack 1 , Clayton R Mulvihill 2 , Stephen J Klippenstein 2 , C Franklin Goldsmith 1
Affiliation
|
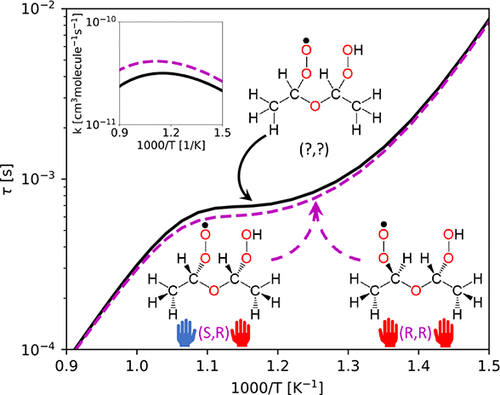
Diastereomers have historically been ignored when building kinetic mechanisms for combustion. Low-temperature oxidation kinetics, which continues to gain interest in both combustion and atmospheric communities, may be affected by the inclusion of diastereomers in radical chain-branching pathways. In this work, key intermediates and transition states lacking stereochemical specification in an existing diethyl ether low-temperature oxidation mechanism were replaced with their diastereomeric counterparts. Rate coefficients for reactions involving diastereomers were computed with ab initio transition state theory master equation calculations. The presence of diastereomers increased rate coefficients by factors of 1.2–1.6 across various temperatures and pressures. Ignition delay simulations incorporating these revised rate coefficients indicate that the diastereomers enhanced the overall reactivity of the mechanism by almost 15% and increased the peak ketohydroperoxide concentration by 30% in the negative temperature coefficient region at combustion-relevant pressures. These results provide an illustrative indication of the important role of stereomeric effects in oxidation kinetics.
中文翻译:

非对映异构体和低温氧化
在建立燃烧动力学机制时,历史上一直忽略非对映异构体。低温氧化动力学继续引起燃烧和大气群落的兴趣,可能会受到自由基链支化途径中非对映异构体的影响。在这项工作中,现有乙醚低温氧化机制中缺乏立体化学规范的关键中间体和过渡态被其非对映异构体替代。涉及非对映异构体的反应的速率系数是用从头过渡态理论主方程计算来计算的。非对映异构体的存在使速率系数在各种温度和压力下增加了 1.2-1.6 倍。结合这些修正的速率系数的点火延迟模拟表明,非对映异构体将机制的整体反应性提高了近 15%,并且在燃烧相关压力下的负温度系数区域中,峰值酮氢过氧化物浓度增加了 30%。这些结果提供了立体异构效应在氧化动力学中的重要作用的说明性指示。
更新日期:2021-09-16
中文翻译:

非对映异构体和低温氧化
在建立燃烧动力学机制时,历史上一直忽略非对映异构体。低温氧化动力学继续引起燃烧和大气群落的兴趣,可能会受到自由基链支化途径中非对映异构体的影响。在这项工作中,现有乙醚低温氧化机制中缺乏立体化学规范的关键中间体和过渡态被其非对映异构体替代。涉及非对映异构体的反应的速率系数是用从头过渡态理论主方程计算来计算的。非对映异构体的存在使速率系数在各种温度和压力下增加了 1.2-1.6 倍。结合这些修正的速率系数的点火延迟模拟表明,非对映异构体将机制的整体反应性提高了近 15%,并且在燃烧相关压力下的负温度系数区域中,峰值酮氢过氧化物浓度增加了 30%。这些结果提供了立体异构效应在氧化动力学中的重要作用的说明性指示。










































 京公网安备 11010802027423号
京公网安备 11010802027423号