当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AFM Identifies a Protein Complex Involved in Pathogen Adhesion Which Ruptures at Three Nanonewtons
Nano Letters ( IF 9.6 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.nanolett.1c02105 Constance Chantraine 1 , Marion Mathelié-Guinlet 1 , Giampiero Pietrocola 2 , Pietro Speziale 2 , Yves F Dufrêne 1
Nano Letters ( IF 9.6 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.nanolett.1c02105 Constance Chantraine 1 , Marion Mathelié-Guinlet 1 , Giampiero Pietrocola 2 , Pietro Speziale 2 , Yves F Dufrêne 1
Affiliation
|
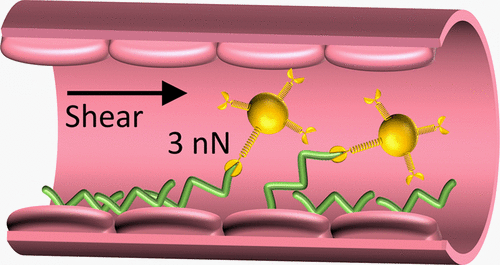
Staphylococci bind to the blood protein von Willebrand Factor (vWF), thereby causing endovascular infections. Whether and how this interaction occurs with the medically important pathogen Staphylococcus epidermidis is unknown. Using single-molecule experiments, we demonstrate that the S. epidermidis protein Aap binds vWF via an ultrastrong force, ∼3 nN, the strongest noncovalent biological bond ever reported, and we show that this interaction is activated by tensile loading, suggesting a catch-bond behavior. Aap–vWF binding involves exclusively the A1 domain of vWF but requires both the A and B domains of Aap, as revealed by inhibition assays using specific monoclonal antibodies. Collectively, our results point to a mechanism where force-induced unfolding of the B repeats activates the A domain of Aap, shifting it from a weak- to a strong-binding state, which then engages into an ultrastrong interaction with vWF A1. This shear-dependent function of Aap offers promise for innovative antistaphylococcal therapies.
中文翻译:

AFM 鉴定了一种与病原体粘附有关的蛋白质复合物,该复合物在三个 Nanonewtons 处破裂
葡萄球菌与血液蛋白血管性血友病因子 (vWF) 结合,从而引起血管内感染。这种相互作用是否与医学上重要的病原体表皮葡萄球菌发生以及如何发生尚不清楚。使用单分子实验,我们证明表皮葡萄球菌蛋白 Aap通过一个超强的力,~3 nN,是迄今为止报道的最强的非共价生物键,我们表明这种相互作用是由拉伸载荷激活的,这表明了一种捕获键的行为。Aap-vWF 结合仅涉及 vWF 的 A1 结构域,但需要 Aap 的 A 和 B 结构域,正如使用特异性单克隆抗体的抑制测定所揭示的那样。总的来说,我们的结果指出了一种机制,即力诱导的 B 重复展开激活 Aap 的 A 结构域,将其从弱结合状态转变为强结合状态,然后与 vWF A1 发生超强相互作用。Aap 的这种剪切依赖性功能为创新的抗葡萄球菌疗法提供了希望。
更新日期:2021-09-22
中文翻译:

AFM 鉴定了一种与病原体粘附有关的蛋白质复合物,该复合物在三个 Nanonewtons 处破裂
葡萄球菌与血液蛋白血管性血友病因子 (vWF) 结合,从而引起血管内感染。这种相互作用是否与医学上重要的病原体表皮葡萄球菌发生以及如何发生尚不清楚。使用单分子实验,我们证明表皮葡萄球菌蛋白 Aap通过一个超强的力,~3 nN,是迄今为止报道的最强的非共价生物键,我们表明这种相互作用是由拉伸载荷激活的,这表明了一种捕获键的行为。Aap-vWF 结合仅涉及 vWF 的 A1 结构域,但需要 Aap 的 A 和 B 结构域,正如使用特异性单克隆抗体的抑制测定所揭示的那样。总的来说,我们的结果指出了一种机制,即力诱导的 B 重复展开激活 Aap 的 A 结构域,将其从弱结合状态转变为强结合状态,然后与 vWF A1 发生超强相互作用。Aap 的这种剪切依赖性功能为创新的抗葡萄球菌疗法提供了希望。











































 京公网安备 11010802027423号
京公网安备 11010802027423号