当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
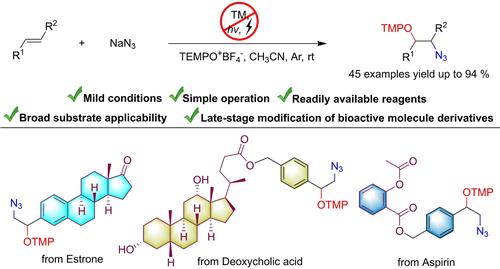
Oxoammonium Salt-Mediated Vicinal Oxyazidation of Alkenes with NaN3: Access to β-Aminooxy Azides
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-01 , DOI: 10.1002/adsc.202100783 Fei Chen 1 , Yu-Ting Tang 1 , Xin-Ru Li 1 , Yan-Yan Duan 1 , Chao-Xing Chen 1 , Yang Zheng 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-01 , DOI: 10.1002/adsc.202100783 Fei Chen 1 , Yu-Ting Tang 1 , Xin-Ru Li 1 , Yan-Yan Duan 1 , Chao-Xing Chen 1 , Yang Zheng 1
Affiliation
|
An approach to the vicinal oxyazidation of alkenes has been achieved under mild and transition metal-free conditions. This method utilizes NaN3 as the azidation agent and 2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate (TEMPO+BF4−) as the single-electron oxidant as well as the oxygen source. By using this protocol, various β-aminooxy azides were synthesized and several complex bioactive molecules were functionalized.
中文翻译:

氧铵盐介导的烯烃与 NaN3 的邻位氧叠氮化:获得 β-氨基氧叠氮化物
在温和且无过渡金属的条件下实现了烯烃邻位氧叠氮化的方法。该方法利用NaN 3作为叠氮化剂和2,2,6,6-四甲基哌啶-1-氧代四氟硼酸铵(TEMPO + BF 4 -)作为单电子氧化剂和氧源。通过使用该协议,合成了各种β-氨基氧叠氮化物, 并对几种复杂的生物活性分子进行了功能化。
更新日期:2021-09-01
中文翻译:

氧铵盐介导的烯烃与 NaN3 的邻位氧叠氮化:获得 β-氨基氧叠氮化物
在温和且无过渡金属的条件下实现了烯烃邻位氧叠氮化的方法。该方法利用NaN 3作为叠氮化剂和2,2,6,6-四甲基哌啶-1-氧代四氟硼酸铵(TEMPO + BF 4 -)作为单电子氧化剂和氧源。通过使用该协议,合成了各种β-氨基氧叠氮化物, 并对几种复杂的生物活性分子进行了功能化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号