European Polymer Journal ( IF 5.8 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.eurpolymj.2021.110745 F. Diot-Néant 1, 2 , L.M.M. Mouterde 1 , J. Couvreur 1 , F. Brunois 1 , S.A. Miller 2 , F. Allais 1, 3
|
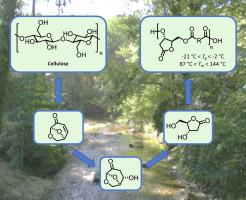
The already reported, yet hazardous, Et3N-catalyzed levoglucosenone (LGO) hydration into 1,6-anhydro-3-deoxy-β-D-erythro-hexo-pyranose-2-ulose (LGO-OH) has been greened up by substituting Et3N with K3PO4 and performing the reaction in H2O. Optimal reaction conditions (K3PO4 (0.05 eq.), [LGO] = 0.08 M, 5 h) not only allowed higher yields, but also limited the homocoupling of LGO, a competitive side reaction. A comparative – yet non-comprehensive and perfectible – Life Cycle Assessment (LCA) using the CML 2002 method highlighted the specific impacts where this revisited synthesis outperformed the Et3N-catalyzed one. 1,6-Anhydro-3-deoxy-β-D-erythro-hexo-pyranose-2-ulose was then subjected to a one-pot catalyst- and organic solvent-free Baeyer-Villiger oxidation/rearrangement, without the need to perform acidic hydrolysis, to access 2-deoxy-D-ribonolactone (HO-HBO, 79% yield). To assess the potential of HO-HBO as monomer for the production of novel bio-based polyesters, the latter was finally polymerized in the presence of aliphatic diacyl chlorides to make a proof-of-concept. Resulting polyesters exhibited promising glass transition temperature (Tg) values between −21 and −2 °C and melting temperatures (Tm) from 87 to 144 °C, demonstrating the potential of HO-HBO for the production of sustainable alternatives to current fossil fuel-based polymers.
中文翻译:

从纤维素衍生的左旋葡萄糖酮 (LGO) 绿色合成 2-脱氧-D-核糖内酯:一种用于新型生物基聚酯的有前途的单体
已报导,但危险,等3的N-催化levoglucosenone(LGO)进水合1,6-脱水-3-脱氧β - D-赤-hexo吡喃糖基-2-酮糖(LGO-OH)已被绿化了通过用 K 3 PO 4取代 Et 3 N并在 H 2 O 中进行反应。最佳反应条件(K 3 PO 4 (0.05 eq.), [ LGO ] = 0.08 M, 5 h)不仅允许更高的产率,而且也限制了LGO的同质耦合,竞争性副反应。使用 CML 2002 方法进行的比较但不全面且可完善的生命周期评估 (LCA) 强调了这种重新审视的合成优于 Et 3 N 催化合成的具体影响。1,6-脱水-3-脱氧β-D-赤-hexo吡喃糖基-2-酮糖然后进行一锅负载催化剂和不含有机溶剂的拜尔-维利格氧化/重排,而无需执行需要酸性水解,得到2-脱氧-D-核糖内酯(HO-HBO,产率79%)。评估HO-HBO的潜力作为生产新型生物基聚酯的单体,后者最终在脂肪族二酰氯的存在下聚合以进行概念验证。所得聚酯表现出有前途的玻璃化转变温度(Ť克)之间的值-21和-2℃和熔化温度(Ť米)从87至144℃,这表明的电位HO-HBO用于生产可持续替代的当前化石燃料基聚合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号