当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed synthesis of α-aryl acetophenones from styryl ethers and aryl diazonium salts via regioselective Heck arylation at room temperature
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-26 , DOI: 10.1039/d1ob01503f Rapelly Venkatesh 1 , Adesh Kumar Singh 1 , Yong Rok Lee 2 , Jeyakumar Kandasamy 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-26 , DOI: 10.1039/d1ob01503f Rapelly Venkatesh 1 , Adesh Kumar Singh 1 , Yong Rok Lee 2 , Jeyakumar Kandasamy 1
Affiliation
|
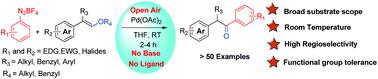
Preparation of α-aryl acetophenones from styryl ethers and aryldiazonium salts is described. The reaction is catalyzed by palladium acetate at room temperature in the absence of ligand and base. The developed method is highly attractive in terms of reaction conditions, substrate scope, functional group tolerance and yields. Synthetic applications of the present method are demonstrated by preparing α-aryl indoles and 3-aryl isocoumarin from styryl ethers.
中文翻译:

钯催化室温下通过区域选择性 Heck 芳基化由苯乙烯基醚和芳基重氮盐合成 α-芳基苯乙酮
描述了从苯乙烯基醚和芳基重氮盐制备 α-芳基苯乙酮。该反应在室温下在没有配体和碱的情况下由乙酸钯催化。所开发的方法在反应条件、底物范围、官能团耐受性和产率方面极具吸引力。本方法的合成应用通过从苯乙烯醚制备 α-芳基吲哚和 3-芳基异香豆素来证明。
更新日期:2021-09-01
中文翻译:

钯催化室温下通过区域选择性 Heck 芳基化由苯乙烯基醚和芳基重氮盐合成 α-芳基苯乙酮
描述了从苯乙烯基醚和芳基重氮盐制备 α-芳基苯乙酮。该反应在室温下在没有配体和碱的情况下由乙酸钯催化。所开发的方法在反应条件、底物范围、官能团耐受性和产率方面极具吸引力。本方法的合成应用通过从苯乙烯醚制备 α-芳基吲哚和 3-芳基异香豆素来证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号