当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 3-nitroindoles by sequential paired electrolysis
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-24 , DOI: 10.1039/d1ob01453f Ashley C Lindsay 1 , Paul A Kilmartin 1 , Jonathan Sperry 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-24 , DOI: 10.1039/d1ob01453f Ashley C Lindsay 1 , Paul A Kilmartin 1 , Jonathan Sperry 1
Affiliation
|
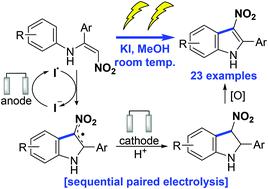
3-Nitroindoles are synthetically versatile intermediates but current methods for the preparation hinder their widespread application. Herein, we report that nitroenamines undergo electrochemical cyclisation to 3-nitroindoles in the presence of potassium iodide. Detailed control experiments and cyclic voltammogram studies infer the reaction proceeds via a sequential paired electrolysis process, beginning with anodic oxidation of iodide (I−) to the iodine radical (I˙), which facilitates cyclisation of the nitroenamine to give a 3-nitroindolinyl radical. Cathodic reduction and protonation generates a 3-nitroindoline that upon oxidation forms the 3-nitroindole.
中文翻译:

顺序配对电解合成3-硝基吲哚
3-硝基吲哚是合成多用途的中间体,但目前的制备方法阻碍了它们的广泛应用。在此,我们报告硝基烯胺在碘化钾存在下电化学环化为 3-硝基吲哚。详细的控制实验和循环伏安图研究推断反应通过顺序配对电解过程进行,从碘化物 (I - ) 阳极氧化为碘自由基 (I˙) 开始,这有助于硝基烯胺环化生成 3-硝基吲哚啉自由基. 阴极还原和质子化生成 3-硝基吲哚,氧化后形成 3-硝基吲哚。
更新日期:2021-09-01
中文翻译:

顺序配对电解合成3-硝基吲哚
3-硝基吲哚是合成多用途的中间体,但目前的制备方法阻碍了它们的广泛应用。在此,我们报告硝基烯胺在碘化钾存在下电化学环化为 3-硝基吲哚。详细的控制实验和循环伏安图研究推断反应通过顺序配对电解过程进行,从碘化物 (I - ) 阳极氧化为碘自由基 (I˙) 开始,这有助于硝基烯胺环化生成 3-硝基吲哚啉自由基. 阴极还原和质子化生成 3-硝基吲哚,氧化后形成 3-硝基吲哚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号