当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptidomimetic nitrile warheads as SARS-CoV-2 3CL protease inhibitors
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2021-08-20 , DOI: 10.1039/d1md00247c Bing Bai 1, 2 , Elena Arutyunova 3, 4 , Muhammad Bashir Khan 3 , Jimmy Lu 2, 4 , Michael A Joyce 2, 4 , Holly A Saffran 2, 4 , Justin A Shields 2, 4 , Appan Srinivas Kandadai 1, 2 , Alexandr Belovodskiy 1, 2 , Mostofa Hena 1, 2 , Wayne Vuong 5 , Tess Lamer 5 , Howard S Young 4 , John C Vederas 5 , D Lorne Tyrrell 1, 2, 4 , M Joanne Lemieux 3, 4 , James A Nieman 1, 2
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2021-08-20 , DOI: 10.1039/d1md00247c Bing Bai 1, 2 , Elena Arutyunova 3, 4 , Muhammad Bashir Khan 3 , Jimmy Lu 2, 4 , Michael A Joyce 2, 4 , Holly A Saffran 2, 4 , Justin A Shields 2, 4 , Appan Srinivas Kandadai 1, 2 , Alexandr Belovodskiy 1, 2 , Mostofa Hena 1, 2 , Wayne Vuong 5 , Tess Lamer 5 , Howard S Young 4 , John C Vederas 5 , D Lorne Tyrrell 1, 2, 4 , M Joanne Lemieux 3, 4 , James A Nieman 1, 2
Affiliation
|
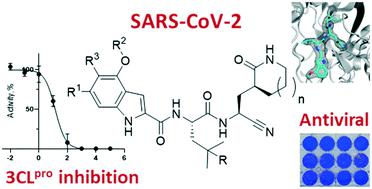
Tragically, the death toll from the COVID-19 pandemic continues to rise, and with variants being observed around the globe new therapeutics, particularly direct-acting antivirals that are easily administered, are desperately needed. Studies targeting the SARS-CoV-2 3CL protease, which is critical for viral replication, with different peptidomimetics and warheads is an active area of research for development of potential drugs. To date, however, only a few publications have evaluated the nitrile warhead as a viral 3CL protease inhibitor, with only modest activity reported. This article describes our investigation of P3 4-methoxyindole peptidomimetic analogs with select P1 and P2 groups with a nitrile warhead that are potent inhibitors of SARS-CoV-2 3CL protease and demonstrate in vitro SARS-CoV-2 antiviral activity. A selectivity for SARS-CoV-2 3CL protease over human cathepsins B, S and L was also observed with the nitrile warhead, which was superior to that with the aldehyde warhead. A co-crystal structure with SARS-CoV-2 3CL protease and a reversibility study indicate that a reversible, thioimidate adduct is formed when the catalytic sulfur forms a covalent bond with the carbon of the nitrile. This effort also identified efflux as a property limiting antiviral activity of these compounds, and together with the positive attributes described these results provide insight for further drug development of novel nitrile peptidomimetics targeting SARS-CoV-2 3CL protease.
中文翻译:

作为 SARS-CoV-2 3CL 蛋白酶抑制剂的拟肽腈弹头
悲惨的是,新冠肺炎 (COVID-19) 大流行造成的死亡人数持续上升,而且随着全球范围内观察到病毒变异,迫切需要新的治疗方法,特别是易于施用的直接作用抗病毒药物。针对 SARS-CoV-2 3CL 蛋白酶(对病毒复制至关重要)的研究以及不同的肽模拟物和弹头是潜在药物开发研究的活跃领域。然而,迄今为止,只有少数出版物评估了腈弹头作为病毒 3CL 蛋白酶抑制剂的作用,并且只报道了适度的活性。本文介绍了我们对 P3 4-甲氧基吲哚拟肽类似物的研究,其中包含带有腈弹头的选定 P1 和 P2 基团,它们是 SARS-CoV-2 3CL 蛋白酶的有效抑制剂,并证明了体外SARS -CoV-2 抗病毒活性。还观察到腈弹头对 SARS-CoV-2 3CL 蛋白酶相对于人组织蛋白酶 B、S 和 L 的选择性,优于醛弹头。与 SARS-CoV-2 3CL 蛋白酶的共晶结构和可逆性研究表明,当催化硫与腈的碳形成共价键时,会形成可逆的硫代亚氨酸盐加合物。这项工作还确定了外排是限制这些化合物抗病毒活性的一种特性,连同所描述的积极属性,这些结果为进一步开发针对 SARS-CoV-2 3CL 蛋白酶的新型腈肽模拟物提供了见解。
更新日期:2021-09-01
中文翻译:

作为 SARS-CoV-2 3CL 蛋白酶抑制剂的拟肽腈弹头
悲惨的是,新冠肺炎 (COVID-19) 大流行造成的死亡人数持续上升,而且随着全球范围内观察到病毒变异,迫切需要新的治疗方法,特别是易于施用的直接作用抗病毒药物。针对 SARS-CoV-2 3CL 蛋白酶(对病毒复制至关重要)的研究以及不同的肽模拟物和弹头是潜在药物开发研究的活跃领域。然而,迄今为止,只有少数出版物评估了腈弹头作为病毒 3CL 蛋白酶抑制剂的作用,并且只报道了适度的活性。本文介绍了我们对 P3 4-甲氧基吲哚拟肽类似物的研究,其中包含带有腈弹头的选定 P1 和 P2 基团,它们是 SARS-CoV-2 3CL 蛋白酶的有效抑制剂,并证明了体外SARS -CoV-2 抗病毒活性。还观察到腈弹头对 SARS-CoV-2 3CL 蛋白酶相对于人组织蛋白酶 B、S 和 L 的选择性,优于醛弹头。与 SARS-CoV-2 3CL 蛋白酶的共晶结构和可逆性研究表明,当催化硫与腈的碳形成共价键时,会形成可逆的硫代亚氨酸盐加合物。这项工作还确定了外排是限制这些化合物抗病毒活性的一种特性,连同所描述的积极属性,这些结果为进一步开发针对 SARS-CoV-2 3CL 蛋白酶的新型腈肽模拟物提供了见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号