当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low Intrinsic Efficacy Alone Cannot Explain the Improved Side Effect Profiles of New Opioid Agonists
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.biochem.1c00466 Edward L Stahl 1 , Laura M Bohn 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.biochem.1c00466 Edward L Stahl 1 , Laura M Bohn 1
Affiliation
|
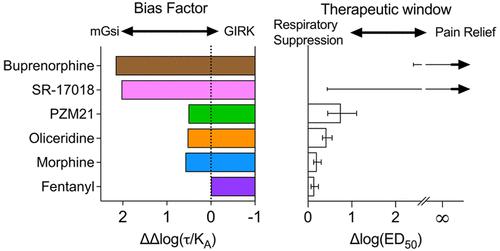
In a recent report in Science Signaling (Gillis, A. , et al. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci. Signaling 2020, 13, eaaz3140 10.1126/scisignal.aaz3140), it was suggested that low intrinsic agonism, and not biased agonism, leads to an improvement in the separation of potency in opioid-induced respiratory suppression versus antinociception. Although many of the compounds that were tested have been shown to display G protein signaling bias in prior publications, the authors conclude that because they cannot detect biased agonism in their cellular signaling studies the compounds are therefore not biased agonists. Rather, they conclude that it is low intrinsic efficacy that leads to the therapeutic window improvement. Intrinsic efficacy is the extent to which an agonist can stimulate a G protein-coupled receptor response in a system, while biased agonism takes into consideration not only the intrinsic efficacy but also the potency of an agonist in an assay. Herein, we have reanalyzed the data presented in the published work (10.1126/scisignal.aaz3140) [including the recent Erratum (10.1126/scisignal.abf9803)] to derive intrinsic efficacy and bias factors as ΔΔlog(τ/KA) and ΔΔlog(Emax/EC50), respectively. On the basis of this reanalysis, the data support the conclusion that biased agonism, favoring G protein signaling, was observed. Moreover, a conservation of rank order intrinsic efficacy was not observed upon comparing responses in each assay, further suggesting that multiple active receptor states were present. These observations agree with prior studies in which oliceridine, PZM21, and SR-17018 were first described as biased agonists with improvement in antinociception over respiratory suppression in mice. Therefore, the data in the Science Signaling paper provide strong corroborating evidence that G protein signaling bias may be a means of improving opioid analgesia while avoiding certain undesirable side effects.
中文翻译:

单独的低内在功效无法解释新型阿片受体激动剂的副作用改善情况
在Science Signaling最近的一份报告中(吉利斯,A. ,等人。G 蛋白活化的低内在功效可以解释新型阿片类激动剂的副作用改善情况。科学。信号 2020 , 13, eaaz3140 10.1126/scisignal.aaz3140),有人提出低内在激动作用,而不是偏向激动作用,导致阿片类药物诱导的呼吸抑制与镇痛作用的效力分离得到改善。尽管许多被测试的化合物已在先前的出版物中显示出 G 蛋白信号传导偏倚,但作者得出结论,由于他们无法在其细胞信号传导研究中检测到偏向激动剂,因此这些化合物不是偏向激动剂。相反,他们得出结论,导致治疗窗口改善的是低内在功效。内在功效是激动剂可以在系统中刺激 G 蛋白偶联受体反应的程度,而偏向激动不仅考虑内在功效,还考虑激动剂在测定中的效力。在此处,K A ) 和 ΔΔlog(Emax/EC 50 )。在此再分析的基础上,数据支持观察到偏向激动、有利于 G 蛋白信号传导的结论。此外,在比较每种测定中的反应时,未观察到等级顺序内在功效的保守性,进一步表明存在多种活性受体状态。这些观察结果与先前的研究一致,其中 oliceridine、PZM21 和 SR-17018 首次被描述为偏向激动剂,在小鼠的呼吸抑制中具有改善的抗伤害感受。因此,Science Signaling论文中的数据提供了强有力的确凿证据,表明 G 蛋白信号偏倚可能是改善阿片类药物镇痛同时避免某些不良副作用的一种手段。
更新日期:2021-09-01
中文翻译:

单独的低内在功效无法解释新型阿片受体激动剂的副作用改善情况
在Science Signaling最近的一份报告中(











































 京公网安备 11010802027423号
京公网安备 11010802027423号