Immunity ( IF 25.5 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.immuni.2021.08.005 Daniele Carvalho Nascimento 1 , Paula Ramos Viacava 2 , Raphael Gomes Ferreira 2 , Marina Alves Damaceno 3 , Annie Rocío Piñeros 2 , Paulo Henrique Melo 2 , Paula Barbim Donate 2 , Juliana Escher Toller-Kawahisa 2 , Daniel Zoppi 4 , Flávio Protásio Veras 2 , Raphael Sanches Peres 2 , Luísa Menezes-Silva 5 , Diego Caetité 2 , Antonio Edson Rocha Oliveira 6 , Ícaro Maia Santos Castro 6 , Gilles Kauffenstein 7 , Helder Imoto Nakaya 8 , Marcos Carvalho Borges 4 , Dario Simões Zamboni 9 , Denise Morais Fonseca 5 , Jonas Augusto Rizzato Paschoal 3 , Thiago Mattar Cunha 2 , Valerie Quesniaux 10 , Joel Linden 11 , Fernando Queíroz Cunha 2 , Bernhard Ryffel 10 , José Carlos Alves-Filho 2
|
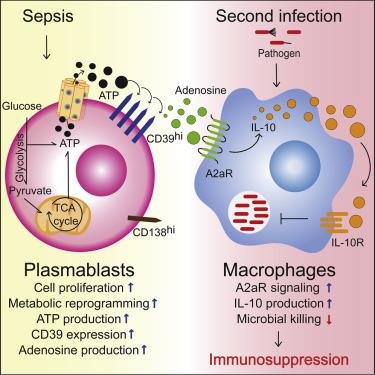
Sepsis results in elevated adenosine in circulation. Extracellular adenosine triggers immunosuppressive signaling via the A2a receptor (A2aR). Sepsis survivors develop persistent immunosuppression with increased risk of recurrent infections. We utilized the cecal ligation and puncture (CLP) model of sepsis and subsequent infection to assess the role of adenosine in post-sepsis immune suppression. A2aR-deficient mice showed improved resistance to post-sepsis infections. Sepsis expanded a subset of CD39hi B cells and elevated extracellular adenosine, which was absent in mice lacking CD39-expressing B cells. Sepsis-surviving B cell-deficient mice were more resistant to secondary infections. Mechanistically, metabolic reprogramming of septic B cells increased production of ATP, which was converted into adenosine by CD39 on plasmablasts. Adenosine signaling via A2aR impaired macrophage bactericidal activity and enhanced interleukin-10 production. Septic individuals exhibited expanded CD39hi plasmablasts and adenosine accumulation. Our study reveals CD39hi plasmablasts and adenosine as important drivers of sepsis-induced immunosuppression with relevance in human disease.
中文翻译:

脓毒症扩大了 CD39+ 浆母细胞群,通过腺苷介导的巨噬细胞抗菌活性抑制促进免疫抑制
脓毒症导致循环中腺苷升高。细胞外腺苷通过 A2a 受体 (A2aR) 触发免疫抑制信号。脓毒症幸存者会出现持续性免疫抑制,复发性感染的风险增加。我们利用败血症和随后感染的盲肠结扎和穿刺 (CLP) 模型来评估腺苷在败血症后免疫抑制中的作用。A2aR 缺陷小鼠对败血症后感染的抵抗力有所提高。脓毒症扩大了 CD39 hi 的一个子集B 细胞和升高的细胞外腺苷,这在缺乏表达 CD39 的 B 细胞的小鼠中是不存在的。脓毒症存活的 B 细胞缺陷小鼠对继发性感染更具抵抗力。从机制上讲,脓毒症 B 细胞的代谢重编程增加了 ATP 的产生,ATP 被浆母细胞上的 CD39 转化为腺苷。通过 A2aR 的腺苷信号传导削弱了巨噬细胞的杀菌活性并增强了白细胞介素 10 的产生。脓毒症个体表现出扩大的 CD39 hi浆母细胞和腺苷积累。我们的研究揭示 CD39 hi浆母细胞和腺苷是脓毒症诱导的免疫抑制的重要驱动因素,与人类疾病相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号