Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.colsurfb.2021.112093 Le Minh Pham 1 , Kishwor Poudel 1 , Cao Dai Phung 1 , Tien Tiep Nguyen 1 , Mahesh Pandit 1 , Hanh Thuy Nguyen 1 , Jae-Hoon Chang 1 , Sung Giu Jin 2 , Jee-Heon Jeong 1 , Sae Kwang Ku 3 , Han-Gon Choi 4 , Chul Soon Yong 1 , Jong Oh Kim 1
|
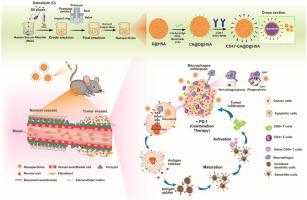
The transmembrane proteins, CD47 and signal-regulatory protein α are overexpressed in cancer cells and macrophages, respectively, and facilitate the escape of cancer cells from macrophage-mediated phagocytosis. The immunomodulatory and targeting properties of CD47, the chemotherapeutic effects of dabrafenib (D), and the anti-programmed death-1 antibodies (PD-1) pave the way for effective chemoimmunomodulation-mediated anticancer combination therapy. In this study, CD47-conjugated, D-loaded human serum albumin (HSA) nanosystems were fabricated by modified nanoparticle albumin-bound technology. Cis-aconityl-PEG-maleimide (CA), an acid-labile linker, was used to conjugate D@HSA and CD47; the resultant CD47-CA@D@HSA exhibited tumor-specificity through receptor targeting, as well as preferential cleavage and drug release in the acidic tumor microenvironment (pH 5) compared to normal physiological pH conditions (pH 6.5, 7.4). The successful preparation of nanosized (∼220 nm), narrowly dispersed (∼0.13) CD47-CA@D@HSA was proven by physicochemical characterization. In vitro and in vivo internalization, accumulation, cytotoxicity, and apoptosis were observed to be higher with CD47-conjugated nanoconstructs, than with free D or non-targeted nanoconstructs. CD47-CA@D@HSA was found to promote the infiltration of cytotoxic T cells and tumor-associated macrophages into tumors and improve in vivo tumor inhibition. Administration in combination with PD-1 further improved antitumor efficacy by promoting immune responses that blocked the immune checkpoint. No signs of toxicity were seen in mice treated with the nanoconstructs; the formulation was, therefore, thought to be biocompatible and as having potential for clinical use. The targeted chemoimmunomodulation achieved by this combination therapy was found to combat major immunosuppressive facets, making it a viable candidate for use in the treatment of cancer.
中文翻译:

用于化学免疫调节的负载达拉非尼、CD47 偶联的人血清白蛋白纳米结构的制备和评价
跨膜蛋白 CD47 和信号调节蛋白 α 分别在癌细胞和巨噬细胞中过表达,并促进癌细胞从巨噬细胞介导的吞噬作用中逃逸。CD47 的免疫调节和靶向特性、dabrafenib (D) 的化学治疗作用和抗程序性死亡 1 抗体 (PD-1) 为有效的化学免疫调节介导的抗癌联合治疗铺平了道路。在这项研究中,CD47 偶联、D 负载人血清白蛋白 (HSA) 纳米系统是通过改进的纳米颗粒白蛋白结合技术制造的。Cis-aconityl-PEG-maleimide (CA),一种酸不稳定接头,用于偶联 D@HSA 和 CD47;由此产生的 CD47-CA@D@HSA 通过受体靶向表现出肿瘤特异性,以及与正常生理 pH 条件(pH 6.5、7.4)相比,酸性肿瘤微环境(pH 5)中的优先裂解和药物释放。物理化学表征证明了纳米尺寸(~220 nm)、窄分散(~0.13)CD47-CA@D@HSA 的成功制备。观察到 CD47 缀合的纳米结构的体外和体内内化、积累、细胞毒性和细胞凋亡高于游离 D 或非靶向纳米结构。发现CD47-CA@D@HSA促进细胞毒性T细胞和肿瘤相关巨噬细胞向肿瘤的浸润并在体内改善肿瘤抑制。通过促进阻断免疫检查点的免疫反应,与 PD-1 联合给药进一步提高了抗肿瘤功效。在用纳米结构治疗的小鼠中没有观察到毒性迹象;因此,该制剂被认为具有生物相容性并具有临床应用潜力。发现通过这种联合疗法实现的靶向化学免疫调节可以对抗主要的免疫抑制方面,使其成为用于治疗癌症的可行候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号