当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deciphering the Origin and Formation of Aminopyrrole Moiety in Kosinostatin Biosynthesis
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-09-01 , DOI: 10.1002/cjoc.202100525 Yu Hu 1 , Qiang Zhou 1, 2 , Zhuan Zhang 1 , Hai‐Xue Pan 1, 3 , Yulia Ilina 4 , Mikko Metsä‐Ketelä 4 , Yasuhiro Igarashi 5 , Gong‐Li Tang 1, 3
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-09-01 , DOI: 10.1002/cjoc.202100525 Yu Hu 1 , Qiang Zhou 1, 2 , Zhuan Zhang 1 , Hai‐Xue Pan 1, 3 , Yulia Ilina 4 , Mikko Metsä‐Ketelä 4 , Yasuhiro Igarashi 5 , Gong‐Li Tang 1, 3
Affiliation
|
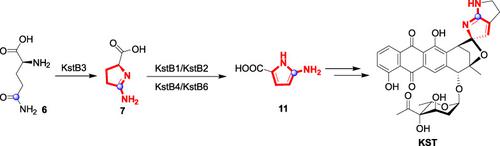
Kosinostatin (KST) contains an uncommon aminopyrrole moiety, whose biosynthesis has remained elusive. Herein, aminopyrrolinic acid, which was generated by an L-ectoine synthase-like enzyme KstB3 via cyclization of L-glutamine, was identified to be the real substrate of adenylation enzyme KstB1. Subsequently, a FAD-dependent dehydrogenase KstB4 along with a transglutaminase-like enzyme KstB6 were also involved in formation of aminopyrrole. These results provided an unusual pathway for 2-aminopyrrole formation in KST biosynthesis.
中文翻译:

破译 Kosinostatin 生物合成中氨基吡咯部分的起源和形成
Kosinostatin (KST) 包含一个罕见的氨基吡咯部分,其生物合成仍然难以捉摸。在此,由 L-四氢嘧啶合酶样酶 KstB3 通过 L-谷氨酰胺环化产生的氨基吡咯啉酸被鉴定为腺苷酸化酶 KstB1 的真正底物。随后,FAD 依赖性脱氢酶 KstB4 和转谷氨酰胺酶样酶 KstB6 也参与了氨基吡咯的形成。这些结果为 KST 生物合成中 2-氨基吡咯的形成提供了一条不寻常的途径。
更新日期:2021-09-01

中文翻译:

破译 Kosinostatin 生物合成中氨基吡咯部分的起源和形成
Kosinostatin (KST) 包含一个罕见的氨基吡咯部分,其生物合成仍然难以捉摸。在此,由 L-四氢嘧啶合酶样酶 KstB3 通过 L-谷氨酰胺环化产生的氨基吡咯啉酸被鉴定为腺苷酸化酶 KstB1 的真正底物。随后,FAD 依赖性脱氢酶 KstB4 和转谷氨酰胺酶样酶 KstB6 也参与了氨基吡咯的形成。这些结果为 KST 生物合成中 2-氨基吡咯的形成提供了一条不寻常的途径。












































 京公网安备 11010802027423号
京公网安备 11010802027423号