当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical α-Trifluoromethoxylation of Ketones under Batch and Flow Conditions by Means of Organic Photoredox Catalysis
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.orglett.1c02494 Thibaut Duhail 1 , Tommaso Bortolato 2 , Javier Mateos 2 , Elsa Anselmi 1, 3 , Benson Jelier 4 , Antonio Togni 4 , Emmanuel Magnier 1 , Guillaume Dagousset 1 , Luca Dell'Amico 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.orglett.1c02494 Thibaut Duhail 1 , Tommaso Bortolato 2 , Javier Mateos 2 , Elsa Anselmi 1, 3 , Benson Jelier 4 , Antonio Togni 4 , Emmanuel Magnier 1 , Guillaume Dagousset 1 , Luca Dell'Amico 2
Affiliation
|
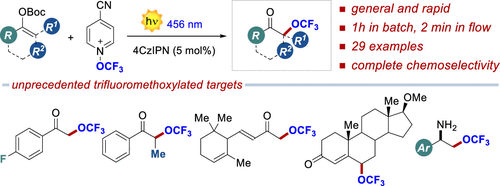
The first light-driven method for the α-trifluoromethoxylation of ketones is reported. Enol carbonates react with N-trifluoromethoxy-4-cyano-pyridinium, using the photoredox catalyst 4-CzIPN under 456 nm irradiation, affording the α-trifluoromethoxy ketones in ≤50% isolated yield and complete chemoselectivity. As shown by 29 examples, the reaction is general and proceeds very rapidly under batch (1 h) and flow conditions (2 min). Diverse product manipulations demonstrate the synthetic potential of the disclosed method in accessing elusive trifluoromethoxylated bioactive ingredients.
中文翻译:

通过有机光氧化还原催化在间歇和流动条件下酮的自由基α-三氟甲氧基化
报道了第一种用于酮的 α-三氟甲氧基化的光驱动方法。烯醇碳酸酯与N-三氟甲氧基-4-氰基-吡啶鎓反应,使用光氧化还原催化剂 4-CzIPN 在 456 nm 照射下,以≤50% 的分离产率和完全化学选择性提供 α-三氟甲氧基酮。如 29 个实施例所示,该反应是通用的,并且在批次(1 小时)和流动条件(2 分钟)下进行得非常快。多样化的产品操作证明了所公开的方法在获取难以捉摸的三氟甲氧基化生物活性成分方面的合成潜力。
更新日期:2021-09-17
中文翻译:

通过有机光氧化还原催化在间歇和流动条件下酮的自由基α-三氟甲氧基化
报道了第一种用于酮的 α-三氟甲氧基化的光驱动方法。烯醇碳酸酯与N-三氟甲氧基-4-氰基-吡啶鎓反应,使用光氧化还原催化剂 4-CzIPN 在 456 nm 照射下,以≤50% 的分离产率和完全化学选择性提供 α-三氟甲氧基酮。如 29 个实施例所示,该反应是通用的,并且在批次(1 小时)和流动条件(2 分钟)下进行得非常快。多样化的产品操作证明了所公开的方法在获取难以捉摸的三氟甲氧基化生物活性成分方面的合成潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号