当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Asymmetric Reduction of Tetrasubstituted Olefin 2,3-Disubstituted Inden-1-ones with Chiral and Regenerable NAD(P)H Model CYNAM
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.orglett.1c02568 Zhou-Hao Zhu 1, 2 , Yi-Xuan Ding 1, 2 , Bo Wu 1 , Yong-Gui Zhou 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.orglett.1c02568 Zhou-Hao Zhu 1, 2 , Yi-Xuan Ding 1, 2 , Bo Wu 1 , Yong-Gui Zhou 1, 3
Affiliation
|
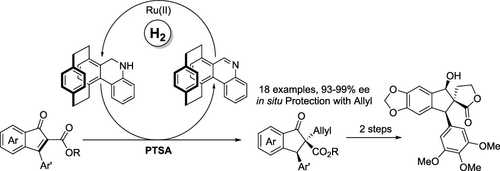
Because of the formidable development of the asymmetric reduction of tetrasubstituted olefins, an effective method is in urgent demand. Herein, through the biomimetic protocol of the coenzyme NAD(P)H, the reduction of tetrasubstituted olefin 2,3-substituted 1H-inden-1-ones has been successfully realized with the catalytic chiral NAD(P)H model CYNAM, which is hard to bring about via the common rhodium or iridium-based catalytic system, producing the corresponding products in good yield (up to 98%) with good enantioselectivity (up to 99% ee). Furthermore, the chiral bioactive molecule can be concisely synthesized from the reduced product.
中文翻译:

具有手性和可再生 NAD(P)H 模型 CYNAM 的四取代烯烃 2,3-二取代 Inden-1-ones 的仿生不对称还原
由于四取代烯烃不对称还原的巨大发展,迫切需要一种有效的方法。在此,通过辅酶 NAD(P)H 的仿生方案,利用催化手性 NAD(P)H 模型 CYNAM 成功实现了四取代烯烃 2,3-取代 1 H -inden-1-ones 的还原。通过普通的铑或铱基催化体系很难实现,以良好的收率(高达 98%)和良好的对映选择性(高达 99% ee)生产相应的产品。此外,可以从还原产物简明地合成手性生物活性分子。
更新日期:2021-09-17
中文翻译:

具有手性和可再生 NAD(P)H 模型 CYNAM 的四取代烯烃 2,3-二取代 Inden-1-ones 的仿生不对称还原
由于四取代烯烃不对称还原的巨大发展,迫切需要一种有效的方法。在此,通过辅酶 NAD(P)H 的仿生方案,利用催化手性 NAD(P)H 模型 CYNAM 成功实现了四取代烯烃 2,3-取代 1 H -inden-1-ones 的还原。通过普通的铑或铱基催化体系很难实现,以良好的收率(高达 98%)和良好的对映选择性(高达 99% ee)生产相应的产品。此外,可以从还原产物简明地合成手性生物活性分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号