当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-Catalyzed Annulation of 2-Biphenylboronic Acid with Diverse Activated Alkenes
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.orglett.1c02597 Bingxian Liu 1 , Lingyun Yang 1 , Zhenzhen Dong 1 , Junbiao Chang 1 , Xingwei Li 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.orglett.1c02597 Bingxian Liu 1 , Lingyun Yang 1 , Zhenzhen Dong 1 , Junbiao Chang 1 , Xingwei Li 1, 2
Affiliation
|
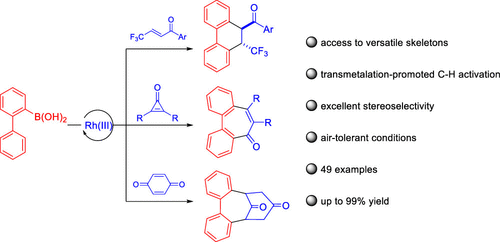
Rhodium(III)-catalyzed annulation of 2-biphenylboronic acids with three classes of activated alkenes has been realized, leading to the synthesis of fused or bridged cyclic skeletons via transmetalation-initiated C–H activation. In the annulative coupling of 2-biphenylboronic acid with a CF3-substituted enone, the bulky cyclopentadienyl ligand (CptBu) in the catalyst proved effective to promote the reductive elimination process prior to protonolysis, affording the [4 + 2] annulated products instead of the simple 1,4-addition product. Seven-membered rings were obtained when disubstituted cyclopropenones were employed. Bridged cycles were isolated from the coupling of 2-biphenylboronic acid with benzoquinones as a result of 2-fold Michael additions. The substrate scopes were found to be broad with up to 99% yield under air-tolerant conditions.
中文翻译:

Rh(III)-催化 2-联苯硼酸与多种活性烯烃的环化
已经实现了铑(III)催化的 2-联苯硼酸与三类活化烯烃的环化,通过金属转移引发的 C-H 活化合成稠合或桥连的环状骨架。在 2-联苯硼酸与 CF 3取代的烯酮的环状偶联中,庞大的环戊二烯基配体 (Cp t Bu) 在催化剂中被证明有效地促进质子分解之前的还原消除过程,提供 [4 + 2] 环化产物而不是简单的 1,4-加成产物。当使用双取代的环丙烯酮时,得到七元环。由于 2 倍迈克尔加成,从 2-联苯硼酸与苯醌的偶联中分离出桥接环。发现底物范围很广,在耐气条件下的产率高达 99%。
更新日期:2021-09-17
中文翻译:

Rh(III)-催化 2-联苯硼酸与多种活性烯烃的环化
已经实现了铑(III)催化的 2-联苯硼酸与三类活化烯烃的环化,通过金属转移引发的 C-H 活化合成稠合或桥连的环状骨架。在 2-联苯硼酸与 CF 3取代的烯酮的环状偶联中,庞大的环戊二烯基配体 (Cp t Bu) 在催化剂中被证明有效地促进质子分解之前的还原消除过程,提供 [4 + 2] 环化产物而不是简单的 1,4-加成产物。当使用双取代的环丙烯酮时,得到七元环。由于 2 倍迈克尔加成,从 2-联苯硼酸与苯醌的偶联中分离出桥接环。发现底物范围很广,在耐气条件下的产率高达 99%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号