当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical Relay Trichloromethylacylation of Alkenes through N-Heterocyclic Carbene Catalysis
Organic Letters ( IF 4.9 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.orglett.1c02639 Mayu Kusakabe 1 , Kazunori Nagao 1 , Hirohisa Ohmiya 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.orglett.1c02639 Mayu Kusakabe 1 , Kazunori Nagao 1 , Hirohisa Ohmiya 1, 2
Affiliation
|
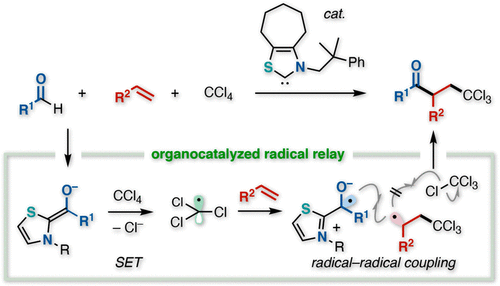
N-Heterocyclic carbene catalysis enabling vicinal trichloromethylacylation of alkenes using tetrachloromethane and aldehydes has been developed. The reaction involves single electron transfer from the enolate form of the Breslow intermediate to tetrachloromethane to generate the persistent Breslow intermediate-derived ketyl radical and a transient trichloromethyl radical. After radical addition of the trichloromethyl radical to an alkene, the prolonged alkyl radical is preferentially captured by the ketyl radical over tetrachloromethane leading to the atom transfer radical addition product.
中文翻译:

N-杂环卡宾催化烯烃自由基三氯甲基酰化反应
已经开发出 N-杂环卡宾催化,能够使用四氯甲烷和醛对烯烃进行邻位三氯甲基酰化。该反应涉及从 Breslow 中间体的烯醇化物形式到四氯甲烷的单电子转移,以生成持久的 Breslow 中间体衍生的羰基自由基和瞬态三氯甲基自由基。在三氯甲基自由基与烯烃的自由基加成之后,延长的烷基自由基优先被酮基自由基捕获而不是四氯甲烷,导致原子转移自由基加成产物。
更新日期:2021-09-17
中文翻译:

N-杂环卡宾催化烯烃自由基三氯甲基酰化反应
已经开发出 N-杂环卡宾催化,能够使用四氯甲烷和醛对烯烃进行邻位三氯甲基酰化。该反应涉及从 Breslow 中间体的烯醇化物形式到四氯甲烷的单电子转移,以生成持久的 Breslow 中间体衍生的羰基自由基和瞬态三氯甲基自由基。在三氯甲基自由基与烯烃的自由基加成之后,延长的烷基自由基优先被酮基自由基捕获而不是四氯甲烷,导致原子转移自由基加成产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号