Neuron ( IF 14.7 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.neuron.2021.08.019 Hailong Yu 1 , Xiao-Chen Bai 1 , Weiwei Wang 1
|
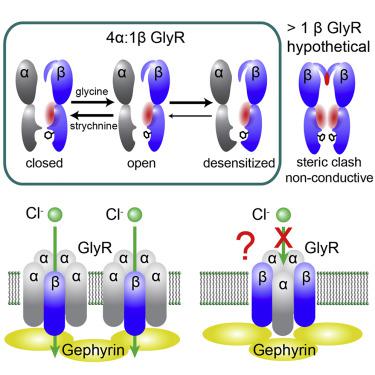
The strychnine-sensitive pentameric glycine receptor (GlyR) mediates fast inhibitory neurotransmission in the mammalian nervous system. Only heteromeric GlyRs mediate synaptic transmission, as they contain the β subunit that permits clustering at the synapse through its interaction with scaffolding proteins. Here, we show that α2 and β subunits assemble with an unexpected 4:1 stoichiometry to produce GlyR with native electrophysiological properties. We determined structures in multiple functional states at 3.6–3.8 Å resolutions and show how 4:1 stoichiometry is consistent with the structural features of α2β GlyR. Furthermore, we show that one single β subunit in each GlyR gives rise to the characteristic electrophysiological properties of heteromeric GlyR, while more β subunits render GlyR non-conductive. A single β subunit ensures a univalent GlyR-scaffold linkage, which means the scaffold alone regulates the cluster properties.
中文翻译:

成人甘氨酸受体亚基组成和结构的表征
马钱子碱敏感的五聚甘氨酸受体 (GlyR) 介导哺乳动物神经系统中的快速抑制性神经传递。只有异聚 GlyRs 介导突触传递,因为它们含有 β 亚基,允许通过与支架蛋白的相互作用在突触处聚集。在这里,我们展示了 α2 和 β 亚基以意想不到的 4:1 化学计量组装,以产生具有天然电生理特性的 GlyR。我们在 3.6-3.8 Å 分辨率下确定了多种功能状态的结构,并展示了 4:1 化学计量与 α2β GlyR 的结构特征是如何一致的。此外,我们表明每个 GlyR 中的一个单个 β 亚基产生异聚 GlyR 的特征电生理特性,而更多的 β 亚基使 GlyR 不导电。







































 京公网安备 11010802027423号
京公网安备 11010802027423号