当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Cross-Coupling of Benzylic C–H Bonds and Azoles with Controlled N-Site Selectivity
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c07117 Si-Jie Chen 1 , Dung L Golden 1 , Shane W Krska 2 , Shannon S Stahl 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c07117 Si-Jie Chen 1 , Dung L Golden 1 , Shane W Krska 2 , Shannon S Stahl 1
Affiliation
|
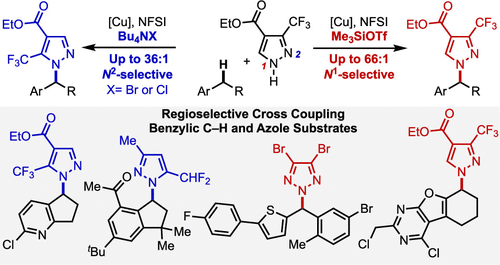
Azoles are important motifs in medicinal chemistry, and elaboration of their structures via direct N–H/C–H coupling could have broad utility in drug discovery. The ambident reactivity of many azoles, however, presents significant selectivity challenges. Here, we report a copper-catalyzed method that achieves site-selective cross-coupling of pyrazoles and other N–H heterocycles with substrates bearing (hetero)benzylic C–H bonds. Excellent N-site selectivity is achieved, with the preferred site controlled by the identity of co-catalytic additives. This cross-coupling strategy features broad scope for both the N–H heterocycle and benzylic C–H coupling partners, enabling application of this method to complex molecule synthesis and medicinal chemistry.
中文翻译:

铜催化的苄基 C-H 键和唑类的交叉偶联,具有受控的 N 位选择性
唑类是药物化学中的重要基序,通过直接 N-H/C-H 偶联来阐述其结构可能在药物发现中具有广泛的用途。然而,许多唑类的环境反应性提出了重大的选择性挑战。在这里,我们报道了一种铜催化方法,该方法实现了吡唑和其他 N-H 杂环与带有(杂)苄基 C-H 键的底物的位点选择性交叉偶联。实现了优异的N位点选择性,优选位点由助催化添加剂的特性控制。这种交叉偶联策略具有广泛的 N-H 杂环和苄基 C-H 偶联伙伴的范围,使得该方法能够应用于复杂的分子合成和药物化学。
更新日期:2021-09-15
中文翻译:

铜催化的苄基 C-H 键和唑类的交叉偶联,具有受控的 N 位选择性
唑类是药物化学中的重要基序,通过直接 N-H/C-H 偶联来阐述其结构可能在药物发现中具有广泛的用途。然而,许多唑类的环境反应性提出了重大的选择性挑战。在这里,我们报道了一种铜催化方法,该方法实现了吡唑和其他 N-H 杂环与带有(杂)苄基 C-H 键的底物的位点选择性交叉偶联。实现了优异的N位点选择性,优选位点由助催化添加剂的特性控制。这种交叉偶联策略具有广泛的 N-H 杂环和苄基 C-H 偶联伙伴的范围,使得该方法能够应用于复杂的分子合成和药物化学。









































 京公网安备 11010802027423号
京公网安备 11010802027423号