当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility and Physical Properties of α-Calcium Sulfate Hemihydrate in NaCl and Glycerol Aqueous Solutions at 303.15, 323.15, and 343.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.jced.1c00214 Bingjie Yu 1 , Shanshan Miao 1 , Manxin Ding 1 , Yongsheng Ren 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.jced.1c00214 Bingjie Yu 1 , Shanshan Miao 1 , Manxin Ding 1 , Yongsheng Ren 1
Affiliation
|
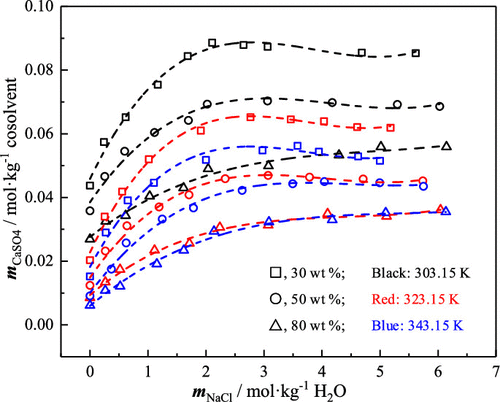
The solubility data of α-calcium sulfate hemihydrate (α-HH) in glycerol and NaCl aqueous solutions containing 30–80 wt % glycerol and 0–6 mol·kg–1 NaCl at 303.15, 323.15, and 343.15 K and atmospheric pressure were determined. The experimental results showed that the solubility of α-HH in this system decreased with the increase of glycerol content. However, with the increase of NaCl concentration, the solubility of α-HH first increased to the maximum and then gradually decreased. The effect of temperature on the solubility of α-HH in glycerol and NaCl aqueous solutions was the same as that in pure water. The physical properties (density and viscosity) of a NaCl–glycerol–H2O system in the saturated state were also determined. At the same time, the relationship between the solubility, density, and viscosity and the molality of NaCl was correlated by a polynomial equation.
中文翻译:

α-半水硫酸钙在 NaCl 和甘油水溶液中的溶解度和物理性质在 303.15、323.15 和 343.15 K
测定了在 303.15、323.15 和 343.15 K 和大气压下,α-硫酸钙半水合物 (α-HH) 在含有 30-80 wt% 甘油和 0-6 mol·kg -1 NaCl 的甘油和 NaCl 水溶液中的溶解度数据. 实验结果表明,α-HH在该体系中的溶解度随着甘油含量的增加而降低。然而,随着NaCl浓度的增加,α-HH的溶解度先增大到最大值,然后逐渐减小。温度对α-HH在甘油和NaCl水溶液中溶解度的影响与在纯水中相同。NaCl-甘油-H 2的物理特性(密度和粘度)还测定了饱和状态下的 O 系统。同时,溶解度、密度和粘度与NaCl的摩尔浓度之间的关系通过多项式方程相关联。
更新日期:2021-10-14
中文翻译:

α-半水硫酸钙在 NaCl 和甘油水溶液中的溶解度和物理性质在 303.15、323.15 和 343.15 K
测定了在 303.15、323.15 和 343.15 K 和大气压下,α-硫酸钙半水合物 (α-HH) 在含有 30-80 wt% 甘油和 0-6 mol·kg -1 NaCl 的甘油和 NaCl 水溶液中的溶解度数据. 实验结果表明,α-HH在该体系中的溶解度随着甘油含量的增加而降低。然而,随着NaCl浓度的增加,α-HH的溶解度先增大到最大值,然后逐渐减小。温度对α-HH在甘油和NaCl水溶液中溶解度的影响与在纯水中相同。NaCl-甘油-H 2的物理特性(密度和粘度)还测定了饱和状态下的 O 系统。同时,溶解度、密度和粘度与NaCl的摩尔浓度之间的关系通过多项式方程相关联。



























 京公网安备 11010802027423号
京公网安备 11010802027423号