当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Behavior of N-Carbobenzyloxyglycine in 14 Individual Solvents at Temperatures Ranging from 283.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.jced.1c00550 Haishuang Huang 1 , Jingxuan Qiu 1 , Hui He 1 , Ying Guo 1, 2 , Shen Hu 1, 2 , Yue Zhao 2, 3 , Peng Wang 1, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.jced.1c00550 Haishuang Huang 1 , Jingxuan Qiu 1 , Hui He 1 , Ying Guo 1, 2 , Shen Hu 1, 2 , Yue Zhao 2, 3 , Peng Wang 1, 3
Affiliation
|
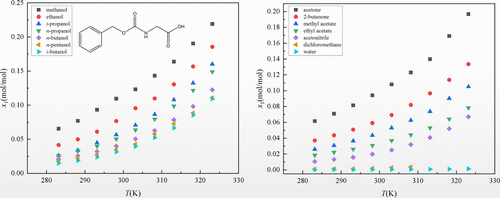
The solubility of N-carbobenzyloxyglycine (Cbz-Gly) in 14 neat solvents including alcohols (methanol, ethanol, i-propanol, n-propanol, n-butanol, i-butanol, and n-pentanol), ketones (acetone and 2-butanone), esters (methyl acetate and ethyl acetate), and other solvents (acetonitrile, dichloromethane, and water) was measured using the static gravimetric method at the temperature interval “T = 283.15–323.15 K” and the pressure “p = 98.8 kPa”. The equilibrated solid phase of Cbz-Gly in the investigated solvent systems was characterized by the powder X-ray diffraction test. The rising temperature shows a positive effect on Cbz-Gly dissolution capacities in the monosolvents, and the following order is obtained: methanol > ethanol > i-propanol > n-propanol > n-butanol > n-pentanol > i-butanol (alcohols) and acetone >2-butanone > methyl acetate > ethyl acetate > acetonitrile > dichloromethane > water (nonalcohols). The above solubility behavior is affected by the mutual constraints of polarity, hydrogen bonding, cohesive energy density, viscosity, and molecular structure. Additionally, two thermodynamic models (the modified Apelblat model and the Yaws model) were used to fit the measured solubility data.
中文翻译:

N-Carbobenzyloxyglycine 在 283.15 至 323.15 K 温度范围内在 14 种单独溶剂中的溶解度行为
的溶解度Ñ -carbobenzyloxyglycine(Cbz基-甘氨酸)在14种整齐溶剂,包括醇类(甲醇,乙醇,我丙醇,Ñ丙醇,Ñ丁醇,我丁醇和Ñ戊醇),酮(丙酮和2-丁酮)、酯(乙酸甲酯和乙酸乙酯)和其他溶剂(乙腈、二氯甲烷和水)使用静态重量法在温度区间“ T = 283.15–323.15 K”和压力“ p”下测量= 98.8 kPa”。Cbz-Gly 在所研究的溶剂系统中的平衡固相通过粉末 X 射线衍射测试进行表征。上升温度上示出了在monosolvents Cbz基-甘氨酸的溶解能力产生积极的影响,并以下列顺序得到:甲醇>乙醇>我丙醇> Ñ丙醇> Ñ丁醇> Ñ戊>我-丁醇(醇类)和丙酮 >2-丁酮 > 乙酸甲酯 > 乙酸乙酯 > 乙腈 > 二氯甲烷 > 水(壬醇类)。上述溶解行为受极性、氢键、内聚能密度、粘度和分子结构等相互制约的影响。此外,两个热力学模型(改进的 Apelblat 模型和 Yaws 模型)用于拟合测量的溶解度数据。
更新日期:2021-10-14
中文翻译:

N-Carbobenzyloxyglycine 在 283.15 至 323.15 K 温度范围内在 14 种单独溶剂中的溶解度行为
的溶解度Ñ -carbobenzyloxyglycine(Cbz基-甘氨酸)在14种整齐溶剂,包括醇类(甲醇,乙醇,我丙醇,Ñ丙醇,Ñ丁醇,我丁醇和Ñ戊醇),酮(丙酮和2-丁酮)、酯(乙酸甲酯和乙酸乙酯)和其他溶剂(乙腈、二氯甲烷和水)使用静态重量法在温度区间“ T = 283.15–323.15 K”和压力“ p”下测量= 98.8 kPa”。Cbz-Gly 在所研究的溶剂系统中的平衡固相通过粉末 X 射线衍射测试进行表征。上升温度上示出了在monosolvents Cbz基-甘氨酸的溶解能力产生积极的影响,并以下列顺序得到:甲醇>乙醇>我丙醇> Ñ丙醇> Ñ丁醇> Ñ戊>我-丁醇(醇类)和丙酮 >2-丁酮 > 乙酸甲酯 > 乙酸乙酯 > 乙腈 > 二氯甲烷 > 水(壬醇类)。上述溶解行为受极性、氢键、内聚能密度、粘度和分子结构等相互制约的影响。此外,两个热力学模型(改进的 Apelblat 模型和 Yaws 模型)用于拟合测量的溶解度数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号