当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Nonradical Oxidation of Sulfonamide Antibiotics with Co(II)-Doped g-C3N4-Activated Peracetic Acid: Role of High-Valent Cobalt–Oxo Species
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.est.1c04091 Banghai Liu 1 , Wanqian Guo 1 , Wenrui Jia 1 , Huazhe Wang 1 , Qishi Si 1 , Qi Zhao 1 , Haichao Luo 1 , Jin Jiang 2 , Nanqi Ren 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.est.1c04091 Banghai Liu 1 , Wanqian Guo 1 , Wenrui Jia 1 , Huazhe Wang 1 , Qishi Si 1 , Qi Zhao 1 , Haichao Luo 1 , Jin Jiang 2 , Nanqi Ren 1
Affiliation
|
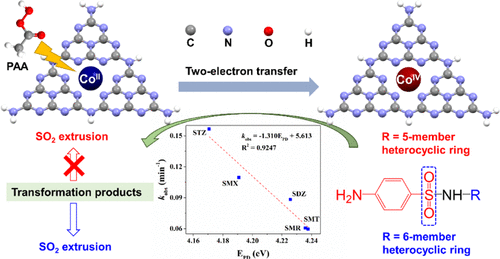
Herein, we report that Co(II)-doped g-C3N4 can efficiently trigger peracetic acid (PAA) oxidation of various sulfonamides (SAs) in a wide pH range. Quite different from the traditional radical-generating or typical nonradical-involved (i.e., singlet oxygenation and mediated electron transfer) catalytic systems, the PAA activation follows a novel nonradical pathway with unprecedented high-valent cobalt–oxo species [Co(IV)] as the dominant reactive species. Our experiments and density functional theory calculations indicate that the Co atom fixated into the nitrogen pots of g-C3N4 serves as the main active site, enabling dissociation of the adsorbed PAA and conversion of the coordinated Co(II) to Co(IV) via a unique two-electron transfer mechanism. Considering Co(IV) to be highly electrophilic in nature, different substituents (i.e., five-membered and six-membered heterocyclic moieties) on the SAs could affect their nucleophilicity, thus leading to the differences in degradation efficiency and transformation pathway. Also, benefiting from the selective oxidation of Co(IV), the established oxidative system exhibits excellent anti-interference capacity and achieves satisfactory decontamination performance under actual water conditions. This study provides a new nonradical approach to degrade SAs by efficiently activating PAA via heterogeneous cobalt-complexed catalysts.
中文翻译:

用 Co(II) 掺杂的 g-C3N4 活化过氧乙酸对磺胺类抗生素进行新型非自由基氧化:高价钴氧物种的作用
在此,我们报告了 Co(II) 掺杂的 gC 3 N 4可以在很宽的 pH 范围内有效地触发各种磺胺 (SA) 的过乙酸 (PAA) 氧化。与传统的自由基生成或典型的非自由基参与(即单线态氧化和介导的电子转移)催化系统完全不同,PAA 活化遵循一种新的非自由基途径,具有前所未有的高价钴 - 羰基化合物 [Co(IV)] 作为占优势的活性物种。我们的实验和密度泛函理论计算表明 Co 原子固定在 gC 3 N 4的氮罐中作为主要的活性位点,通过独特的双电子转移机制使吸附的 PAA 解离并将配位的 Co(II) 转化为 Co(IV)。考虑到 Co(IV) 在性质上具有高度亲电性,SAs 上的不同取代基(即五元和六元杂环部分)会影响它们的亲核性,从而导致降解效率和转化途径的差异。此外,受益于 Co(IV) 的选择性氧化,所建立的氧化体系表现出优异的抗干扰能力,并在实际水条件下实现了令人满意的去污性能。该研究提供了一种新的非自由基方法,通过多相钴络合催化剂有效活化 PAA 来降解 SA。
更新日期:2021-09-21
中文翻译:

用 Co(II) 掺杂的 g-C3N4 活化过氧乙酸对磺胺类抗生素进行新型非自由基氧化:高价钴氧物种的作用
在此,我们报告了 Co(II) 掺杂的 gC 3 N 4可以在很宽的 pH 范围内有效地触发各种磺胺 (SA) 的过乙酸 (PAA) 氧化。与传统的自由基生成或典型的非自由基参与(即单线态氧化和介导的电子转移)催化系统完全不同,PAA 活化遵循一种新的非自由基途径,具有前所未有的高价钴 - 羰基化合物 [Co(IV)] 作为占优势的活性物种。我们的实验和密度泛函理论计算表明 Co 原子固定在 gC 3 N 4的氮罐中作为主要的活性位点,通过独特的双电子转移机制使吸附的 PAA 解离并将配位的 Co(II) 转化为 Co(IV)。考虑到 Co(IV) 在性质上具有高度亲电性,SAs 上的不同取代基(即五元和六元杂环部分)会影响它们的亲核性,从而导致降解效率和转化途径的差异。此外,受益于 Co(IV) 的选择性氧化,所建立的氧化体系表现出优异的抗干扰能力,并在实际水条件下实现了令人满意的去污性能。该研究提供了一种新的非自由基方法,通过多相钴络合催化剂有效活化 PAA 来降解 SA。



























 京公网安备 11010802027423号
京公网安备 11010802027423号