当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Access to Benzocycles through Copper-Catalyzed Borylative Cyclizations
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-31 , DOI: 10.1002/adsc.202100812 Jaesook Yun 1 , Wan Yoon 2 , Jung Tae Han 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-31 , DOI: 10.1002/adsc.202100812 Jaesook Yun 1 , Wan Yoon 2 , Jung Tae Han 1
Affiliation
|
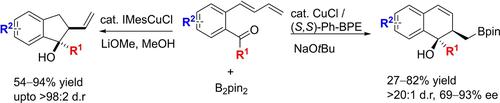
A copper-catalyzed chemodivergent approach to five- and six-membered benzocycles from dienyl arenes tethered with a ketone has been developed. Through proper choice of coordinating ligands and catalytic conditions, copper-catalyzed borylative cyclization of a single dienyl arene can be diverted to two different pathways, leading to indanols and dihydronaphthalenols with high stereoselectivity. The chiral bidentate bisphosphine ligand (S,S)-Ph-BPE was optimal for asymmetric copper-allyl addition to a tethered ketone via a boat-like transition state, whereas NHC ligands led to boro-allyl addition producing indanols with high diastereoselectivity.
中文翻译:

通过铜催化硼酸化环化不同途径获得苯环
已经开发了一种铜催化化学发散方法,用于从与酮相连的二烯基芳烃制备五元和六元苯并环。Through proper choice of coordinating ligands and catalytic conditions, copper-catalyzed borylative cyclization of a single dienyl arene can be diverted to two different pathways, leading to indanols and dihydronaphthalenols with high stereoselectivity. 手性双齿双膦配体 ( S , S )-Ph-BPE 最适用于通过船状过渡态将铜烯丙基不对称加成到系链酮上,而 NHC 配体导致硼烯丙基加成产生具有高非对映选择性的茚满醇。
更新日期:2021-11-09
中文翻译:

通过铜催化硼酸化环化不同途径获得苯环
已经开发了一种铜催化化学发散方法,用于从与酮相连的二烯基芳烃制备五元和六元苯并环。Through proper choice of coordinating ligands and catalytic conditions, copper-catalyzed borylative cyclization of a single dienyl arene can be diverted to two different pathways, leading to indanols and dihydronaphthalenols with high stereoselectivity. 手性双齿双膦配体 ( S , S )-Ph-BPE 最适用于通过船状过渡态将铜烯丙基不对称加成到系链酮上,而 NHC 配体导致硼烯丙基加成产生具有高非对映选择性的茚满醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号