当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influences of nano-effect on the thermodynamic properties of solid–liquid interfaces: theoretical and experimental researches
CrystEngComm ( IF 2.6 ) Pub Date : 2021-08-10 , DOI: 10.1039/d1ce00906k Huijuan Duan 1 , Zuohui Cheng 1 , Yongqiang Xue 2 , Jinzhong Zhao 1 , Meihong Yang 1 , Zixiang Cui 2 , Wenmei Gao 1 , Shiyao Wang 1
CrystEngComm ( IF 2.6 ) Pub Date : 2021-08-10 , DOI: 10.1039/d1ce00906k Huijuan Duan 1 , Zuohui Cheng 1 , Yongqiang Xue 2 , Jinzhong Zhao 1 , Meihong Yang 1 , Zixiang Cui 2 , Wenmei Gao 1 , Shiyao Wang 1
Affiliation
|
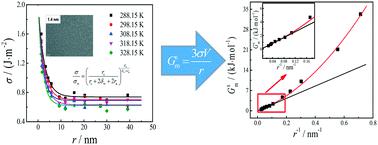
The unique physical and chemical properties of nanoparticles are closely related to the interfacial properties, which depend on the nano-effect. Herein, we theoretically analyzed the relationships of interfacial thermodynamic properties with particle size and discussed the influencing mechanism and regularities of nano-effect on interfacial thermodynamic properties. Experimentally, nano-Ag with different radii (1.4 nm to 39.1 nm) was prepared by the liquid phase reduction method. The interfacial tension, its temperature coefficient, and the interfacial thermodynamic properties of the interface between nano-Ag and its electrolyte solution were determined by an electrochemical method. Both the theoretical analysis and the experimental results show that nano-effect has a great influence on the interfacial properties of nanoparticles. Nano-effect mainly consists of interfacial area effect and interfacial tension effect. With the decreasing particle size, nano-effect increases; interfacial Gibbs energy, interfacial internal energy, interfacial enthalpy, and interfacial entropy increase. When the radius exceeds 10 nm, the interfacial area effect is the crucial influencing factor for interfacial thermodynamic properties, and there exist linear relations of these interfacial thermodynamic properties and the reciprocal of radius. When the radius is less than 10 nm, both interfacial area effect and interfacial tension effect (including the effect of interfacial tension and its temperature coefficient) influence interfacial thermodynamic properties, resulting in deviations from the linear relationships, and the smaller the particle size, the more obvious the deviation. In addition, for nano-Ag, when the radius is reduced to 1.5 nm, the influence of interfacial area effect and interfacial tension effect on interfacial thermodynamic properties are nearly equal, and interfacial tension effect will be crucial with continuing decrease of the particle size. This research can provide significant fundamental insight into size-dependent interfacial properties and explain and predict the regularities of the effect of particle size on thermodynamic properties in various physical and chemical processes.
中文翻译:

纳米效应对固液界面热力学性质的影响:理论和实验研究
纳米粒子独特的物理和化学性质与依赖于纳米效应的界面性质密切相关。在此,我们从理论上分析了界面热力学性质与粒径的关系,并讨论了纳米效应对界面热力学性质的影响机制和规律。实验上,采用液相还原法制备了不同半径(1.4 nm 至 39.1 nm)的纳米银。通过电化学方法测定了纳米银与其电解质溶液界面的界面张力、温度系数和界面热力学性质。理论分析和实验结果均表明纳米效应对纳米粒子的界面性质有很大影响。纳米效应主要包括界面面积效应和界面张力效应。随着粒径的减小,纳米效应增强;界面吉布斯能、界面内能、界面焓和界面熵增加。当半径超过10 nm时,界面面积效应是影响界面热力学性质的关键因素,这些界面热力学性质与半径的倒数存在线性关系。当半径小于 10 nm 时,界面面积效应和界面张力效应(包括界面张力效应及其温度系数)都会影响界面热力学性质,导致偏离线性关系,粒径越小,偏差比较明显。此外,对于纳米Ag,当半径减小到1.5 nm时,界面面积效应和界面张力效应对界面热力学性质的影响几乎相等,随着粒径的不断减小,界面张力效应将变得至关重要。这项研究可以提供对尺寸相关界面特性的重要基础见解,并解释和预测粒径对各种物理和化学过程中热力学特性影响的规律。
更新日期:2021-09-01
中文翻译:

纳米效应对固液界面热力学性质的影响:理论和实验研究
纳米粒子独特的物理和化学性质与依赖于纳米效应的界面性质密切相关。在此,我们从理论上分析了界面热力学性质与粒径的关系,并讨论了纳米效应对界面热力学性质的影响机制和规律。实验上,采用液相还原法制备了不同半径(1.4 nm 至 39.1 nm)的纳米银。通过电化学方法测定了纳米银与其电解质溶液界面的界面张力、温度系数和界面热力学性质。理论分析和实验结果均表明纳米效应对纳米粒子的界面性质有很大影响。纳米效应主要包括界面面积效应和界面张力效应。随着粒径的减小,纳米效应增强;界面吉布斯能、界面内能、界面焓和界面熵增加。当半径超过10 nm时,界面面积效应是影响界面热力学性质的关键因素,这些界面热力学性质与半径的倒数存在线性关系。当半径小于 10 nm 时,界面面积效应和界面张力效应(包括界面张力效应及其温度系数)都会影响界面热力学性质,导致偏离线性关系,粒径越小,偏差比较明显。此外,对于纳米Ag,当半径减小到1.5 nm时,界面面积效应和界面张力效应对界面热力学性质的影响几乎相等,随着粒径的不断减小,界面张力效应将变得至关重要。这项研究可以提供对尺寸相关界面特性的重要基础见解,并解释和预测粒径对各种物理和化学过程中热力学特性影响的规律。











































 京公网安备 11010802027423号
京公网安备 11010802027423号