Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.bioorg.2021.105318 Amal A M Eissa 1 , Kholoud F M Aljamal 1 , Hany S Ibrahim 2 , Heba Abdelrasheed Allam 1
|
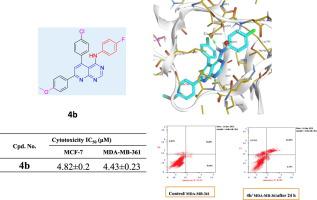
The present study describes the synthesis of three series of 4-substituted pyridopyrimidin derivatives 4a-h, 5a-d. 6a-d, starting from 2-amino-6-(4-methoxyphenyl)-4-(4-(substituted) phenyl)nicotinonitrile 2a-d via the reaction with N,N-dimethyl-N-' substituted phenyl formimidamide to obtain 4a-h or with either phenyl isothiocyanate 1:1 and 1:2 to obtain 5a-d, 6a-d respectively. The synthesized compounds were evaluated for their effectiveness as EGFR inhibitors against Gefitinib. Six compounds; 4b,g,h, 5c and 6a,d prompted significantly higher EGFR inhibitory activity relative to that of Gefitinib. While two compounds 4d and 4f showed IC50 values non-significantly different from that of the reference drug. Furthermore, compounds 4a, 4 h, 6a and 6d were chosen to be assessed in vitro for their cytotoxicity against two EGFR-overexpressing cell lines; two human cancer cell lines namely: MCF7 and MDA-MB-361. Moreover, cell cycle analysis and apoptotic assay was applied for compound 4b that showed most potent inhibitory activity on EGFR, and the highest cytotoxicity against MCF7 and MDA-MB-361, where cell cycle arrest was achieved at pre G and S phases with increased apoptosis. Additionally, a molecular docking study was achieved to inspect the interaction of this compound with the active site of EGFR-TK.
中文翻译:

设计和合成具有锚定非共面芳香族延伸的 EGFR 抑制活性的新型吡啶并嘧啶衍生物
本研究描述了三个系列的 4-取代吡啶并嘧啶衍生物4a-h、5a-d的合成。6a-d,由2-氨基-6-(4-甲氧基苯基)-4-(4-(取代)苯基)烟腈2a-d与N,N-二甲基-N- '取代苯基甲酰亚胺反应得到4a-h或与异硫氰酸苯酯 1:1 和 1:2 分别得到5a-d和6a-d。对合成的化合物作为针对吉非替尼的EGFR抑制剂的有效性进行了评估。六种化合物;4b,g,h, 5c和6a,d与吉非替尼相比,EGFR抑制活性显着提高。虽然两种化合物4d和4f 的IC 50值与参考药物的IC 50值没有显着差异。此外,选择化合物4a、4h、6a和6d在体外评估它们对两种EGFR 过表达细胞系的细胞毒性;两种人类癌细胞系,即:MCF7和MDA-MB - 361. 此外,对化合物 4b 进行细胞周期分析和细胞凋亡测定,该化合物对 EGFR 显示出最有效的抑制活性,对 MCF7 和 MDA-MB-361 的细胞毒性最高,其中细胞周期停滞在前 G 和 S 期,细胞凋亡增加. 此外,还进行了一项分子对接研究,以检查该化合物与 EGFR-TK 活性位点的相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号