Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.bioorg.2021.105319 Wenliang Wang 1 , Denghui Gao 2 , Qiancheng Zheng 2 , Xi Zhao 2 , Risong Na 1 , Xinsheng Wan 1 , Qing X Li 3
|
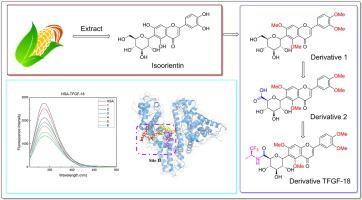
Isoorientin is a C-glycosyl flavone with a wide range of health beneficial effects and inhibits glycogen synthase kinase 3β (GSK-3β) potentially against Alzheimer’s disease. Its semi-synthetic derivatives have greater potency than isoorientin. The present study was aimed to determine the mechanism of interactions of isoorientin and its derivatives with human serum albumin (HSA) using multi-spectroscopic, microscale thermophoresis (MST) and computational studies. Spectra of steady-state fluorescence, UV–Vis, and time-resolved fluorescence indicated that isoorientin and its derivatives quenched the intrinsic fluorescence of HSA through a static quenching process. Isoorientin and its derivatives had a moderate affinity with HSA (Ka 7.7–14.9 × 104 M−1). The binding process was accompanied by an exothermic phenomenon, ΔG° of HSA-isoorientin and its derivatives systems were calculated as from −29.51 kJ mol−1 to −27.87 kJ mol−1. Displacement experiments with site-specific markers revealed that isoorientin and its derivatives bind to HSA at site II (subdomain IIIA) only. A reduction in the α-helical content of HSA-isoorientin and its derivatives complex was observed, because the conformational changes was structurally perturbed by the hydrophilic groups of the compounds. Further molecular modeling studies confirmed that the binding of isoorientin and its derivatives to the site II via hydrophobic interaction. The MST results confirmed the interactions between HSA and the compounds of interest. The esterase-like assay studies indicated that isoorientin and its derivatives shared the same binding site in HSA, and their induced structural changes of HSA may have been caused by partial unfolding of HSA. This work helps to understand transport, distribution, bioactivity, and design of flavonoid-based GSK-3β inhibitors.
中文翻译:

异东方素及其半合成类似物与人血清白蛋白的相互作用
Isoorientin 是一种 C-糖基黄酮,具有广泛的健康益处,可抑制糖原合酶激酶 3β (GSK-3β),可能对抗阿尔茨海默病。它的半合成衍生物比异东方素具有更大的效力。本研究旨在使用多光谱、微尺度热泳 (MST) 和计算研究确定异东方素及其衍生物与人血清白蛋白 (HSA) 的相互作用机制。稳态荧光、UV-Vis 和时间分辨荧光光谱表明异定向素及其衍生物通过静态淬灭过程淬灭了 HSA 的固有荧光。异东方素及其衍生物与 HSA ( K a 7.7–14.9 × 10 4 M -1)。结合过程伴随着放热现象,HSA-isoorientin 及其衍生物系统的ΔG °计算为-29.51 kJ mol -1到-27.87 kJ mol -1. 位点特异性标记的置换实验表明,异定向素及其衍生物仅在位点 II(亚域 IIIA)与 HSA 结合。观察到 HSA-isoorientin 及其衍生物复合物的 α-螺旋含量减少,因为化合物的亲水基团在结构上扰乱了构象变化。进一步的分子建模研究证实了异定向素及其衍生物通过疏水相互作用与位点 II 的结合。MST 结果证实了 HSA 与目标化合物之间的相互作用。酯酶样测定研究表明异定向素及其衍生物在 HSA 中具有相同的结合位点,它们诱导的 HSA 结构变化可能是由 HSA 的部分解折叠引起的。这项工作有助于了解运输、分布、生物活性、











































 京公网安备 11010802027423号
京公网安备 11010802027423号