Biochimie ( IF 3.3 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.biochi.2021.08.008 Aleksandr Viktorovich Protasov 1 , Olga Alexandrovna Mirgorodskaya 1 , Yuri Petrovich Kozmin 2 , Johan Gobom 3
|
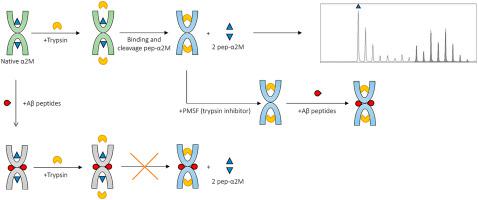
We used MALDI-MS to study the interaction of amyloid β (Aβ) peptides with alpha-2-macroglobulin (α2M). The binding of amyloid beta (Aβ) peptides to alpha-2-macroglobulin (α2M) was found to inhibit the ability of trypsin to cleave out the peptide α2M 705–715 (Pep-α2M) from α2M. This was observed with both purified α2M and α2M in human serum. We found that Aβ 1–38, Aβ1-40, and Aβ 1–42, all inhibit the interaction of α2M with trypsin, with inhibition rate independent of the length of the Aβ peptide. Further, we show that for complete inhibition, two peptide molecules must be attached to one α2M molecule; one for each of its two subunits. A region was revealed within the Aβ sequence, in which proteolytic cleavage (Lys-28) and oxidation (Met-35) lead to a loss of their ability to inhibit the interaction of trypsin with α2M. Furthermore, we show that after the formation of a trypsin complex with α2M and cleavage of α2M to produce the α2M 705–715, Aβ peptides continue to bind to the protein in the same proportions. However, Aβ peptides treated with DMSO lost their ability to bind to α2M and thereby to inhibit the interaction of trypsin with α2M. While maintaining their primary structure, such an effect can be explained only by conformational changes in the peptides, suggesting the possibility to use our analytical approach to distinguish between conformational isomers of Aβ peptides.
中文翻译:

研究淀粉样蛋白β肽与人α-2-巨球蛋白相互作用的质谱方法
我们使用 MALDI-MS 研究淀粉样蛋白 β (Aβ) 肽与 α-2-巨球蛋白 (α2M) 的相互作用。发现淀粉样蛋白 β (Aβ) 肽与 α-2-巨球蛋白 (α2M) 的结合可抑制胰蛋白酶从 α2M 中切割出肽 α2M 705–715 (Pep-α2M) 的能力。这在人血清中用纯化的 α2M 和 α2M 都观察到。我们发现 Aβ 1-38、Aβ1-40 和 Aβ 1-42 均能抑制 α2M 与胰蛋白酶的相互作用,其抑制率与 Aβ 肽的长度无关。此外,我们表明,为了完全抑制,两个肽分子必须连接到一个 α2M 分子上。其两个亚基中的每一个。在 Aβ 序列中发现了一个区域,其中蛋白水解切割 (Lys-28) 和氧化 (Met-35) 导致它们丧失抑制胰蛋白酶与 α2M 相互作用的能力。此外,我们表明,在与 α2M 形成胰蛋白酶复合物并裂解 α2M 以产生 α2M 705-715 后,Aβ 肽继续以相同的比例与蛋白质结合。然而,用 DMSO 处理的 Aβ 肽失去了与 α2M 结合的能力,从而抑制了胰蛋白酶与 α2M 的相互作用。在保持其一级结构的同时,这种效应只能通过肽的构象变化来解释,这表明可以使用我们的分析方法来区分 Aβ 肽的构象异构体。










































 京公网安备 11010802027423号
京公网安备 11010802027423号