Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.jorganchem.2021.122068 Zhifo Guo 1 , Xiangyang Lei 1
|
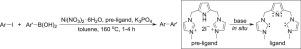
A new catalytic system with Ni(NO3)2·6H2O as the catalyst and a pincer pyrrole-functionalized N-heterocyclic carbene as the ligand was employed in the Suzuki-Miyaura cross-coupling reactions of aryl iodides with arylboronic acids. With 5 mol% catalyst, the catalytic reactions proceeded at 160 °C, giving coupling products in isolated yields of up to 94% in short reaction times (1-4 h). The system worked efficiently with aryl iodides bearing electron-donating or electron-withdrawing groups and arylboronic acids with electron-donating groups. Steric effects were observed for both aryl iodides and arylboronic acids. It is proposed that the reactions underwent a Ni(I)/Ni(III) catalytic cycle.
中文翻译:

以钳形吡咯官能化 N-杂环卡宾为配体的新型镍基催化体系,用于铃木-宫浦交叉偶联反应
以Ni(NO 3 ) 2 ·6H 2 O为催化剂,以钳形吡咯官能化的N-杂环卡宾为配体的新型催化体系用于芳基碘与芳基硼酸的Suzuki-Miyaura交叉偶联反应。使用 5 mol% 的催化剂,催化反应在 160 °C 下进行,在短反应时间(1-4 小时)内以高达 94% 的分离产率得到偶联产物。该系统与带有给电子或吸电子基团的芳基碘化物和带有给电子基团的芳基硼酸一起有效地工作。对芳基碘化物和芳基硼酸均观察到空间效应。提出该反应经历了 Ni(I)/Ni(III) 催化循环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号