当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Assessment of the Dry Reforming of Methane over a Ni–La2O3 Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-01 , DOI: 10.1021/acscatal.1c02631 Víctor Stivenson Sandoval-Bohórquez 1 , Edgar M. Morales-Valencia 1 , Carlos O. Castillo-Araiza 2 , Luz M. Ballesteros-Rueda 1 , Víctor G. Baldovino-Medrano 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-01 , DOI: 10.1021/acscatal.1c02631 Víctor Stivenson Sandoval-Bohórquez 1 , Edgar M. Morales-Valencia 1 , Carlos O. Castillo-Araiza 2 , Luz M. Ballesteros-Rueda 1 , Víctor G. Baldovino-Medrano 1, 3
Affiliation
|
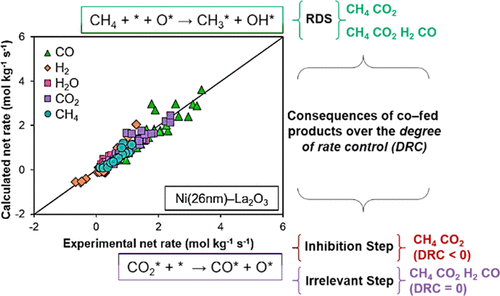
The dry reforming of methane is a promising technology for the abatement of CH4 and CO2. Ni–La2O3 catalysts are characterized by their long-term stability (100 h) when tested at full conversion. The kinetics of dry reforming over these types of catalysts has been studied using both power-law and Langmuir–Hinshelwood-based approaches. However, these studies typically deal with fitting the net CH4 rate, hence disregarding competing and parallel surface processes and the different possible configurations of the active surface. In this work, we synthesized a Ni–La2O3 catalyst and tested six Langmuir–Hinshelwood mechanisms considering both single and dual active sites for assessing the kinetics of dry reforming and the competing reverse water–gas shift reaction and investigated the performance of the derived kinetic models. In doing this, it was found that: (1) all of the net rates were better fitted by a single-site model that considered that the first C–H bond cleavage in methane occurred over a metal–oxygen pair site; (2) this model predicted the existence of a nearly saturated nickel surface with chemisorbed oxygen adatoms derived from the CO2 dissociation; (3) the CO2dissociation can either be an inhibitory or an irrelevant step, and it can also modify the apparent activation energy for CH4 activation. These findings contribute to a better understanding of the dry reforming reaction’s kinetics and provide a robust kinetic model for the design and scale-up of the process.
中文翻译:

Ni-La2O3 催化剂上甲烷干重整的动力学评估
甲烷干法重整是一种很有前景的CH 4和CO 2减排技术。Ni-La 2 O 3催化剂在完全转化率测试时具有长期稳定性(100 小时)的特点。已经使用幂律和基于 Langmuir-Hinshelwood 的方法研究了这些类型催化剂的干重整动力学。然而,这些研究通常涉及拟合净 CH 4速率,因此忽略了竞争和平行表面过程以及活性表面的不同可能配置。在这项工作中,我们合成了 Ni-La 2 O 3催化剂并测试了六种 Langmuir-Hinshelwood 机制,同时考虑了单活性位点和双活性位点,以评估干重整和竞争性反向水煤气变换反应的动力学,并研究了衍生动力学模型的性能。在这样做时,发现:(1)所有净速率都更适合单中心模型,该模型认为甲烷中的第一个 C-H 键断裂发生在金属-氧对位点上;(2) 该模型预测存在接近饱和的镍表面,并具有源自 CO 2解离的化学吸附氧原子;(3) CO 2解离可以是一个抑制步骤,也可以是一个无关步骤,它也可以改变 CH 4的表观活化能激活。这些发现有助于更好地了解干重整反应的动力学,并为该过程的设计和放大提供可靠的动力学模型。
更新日期:2021-09-17
中文翻译:

Ni-La2O3 催化剂上甲烷干重整的动力学评估
甲烷干法重整是一种很有前景的CH 4和CO 2减排技术。Ni-La 2 O 3催化剂在完全转化率测试时具有长期稳定性(100 小时)的特点。已经使用幂律和基于 Langmuir-Hinshelwood 的方法研究了这些类型催化剂的干重整动力学。然而,这些研究通常涉及拟合净 CH 4速率,因此忽略了竞争和平行表面过程以及活性表面的不同可能配置。在这项工作中,我们合成了 Ni-La 2 O 3催化剂并测试了六种 Langmuir-Hinshelwood 机制,同时考虑了单活性位点和双活性位点,以评估干重整和竞争性反向水煤气变换反应的动力学,并研究了衍生动力学模型的性能。在这样做时,发现:(1)所有净速率都更适合单中心模型,该模型认为甲烷中的第一个 C-H 键断裂发生在金属-氧对位点上;(2) 该模型预测存在接近饱和的镍表面,并具有源自 CO 2解离的化学吸附氧原子;(3) CO 2解离可以是一个抑制步骤,也可以是一个无关步骤,它也可以改变 CH 4的表观活化能激活。这些发现有助于更好地了解干重整反应的动力学,并为该过程的设计和放大提供可靠的动力学模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号