当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Analysis Uncovers Hidden Autocatalysis and Inhibition Pathways in Titanium(III)-Catalyzed Ketone-Nitrile Couplings
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-08-31 , DOI: 10.1021/acscatal.1c02870 Sara L. Younas 1 , Jan Streuff 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-08-31 , DOI: 10.1021/acscatal.1c02870 Sara L. Younas 1 , Jan Streuff 1, 2
Affiliation
|
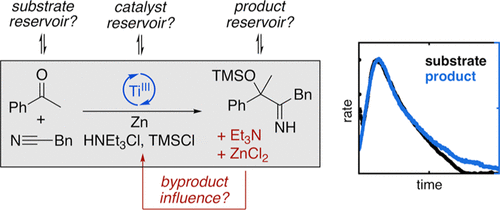
Through kinetic analysis, we demonstrate that the formation of catalyst and substrate reservoirs plays a crucial role in the titanium(III)-catalyzed ketone-nitrile coupling reaction. Together with the insight from previous studies, a kinetic model is assembled that reflects the observed influence of ZnCl2 and Et3N byproducts as well as the hydrochloride additive, which is employed frequently in titanium(III) catalysis. We further propose for the Cp2TiCl2-catalyzed reaction that the acceleration phase of the reaction originates from a hidden autocatalytic cycle, which leads to a change in the rate-determining step over the course of the reaction.
中文翻译:

动力学分析揭示了钛 (III) 催化的酮-腈偶联物中隐藏的自催化和抑制途径
通过动力学分析,我们证明催化剂和底物储层的形成在钛 (III) 催化的酮腈偶联反应中起着至关重要的作用。结合先前研究的见解,组装了一个动力学模型,该模型反映了观察到的 ZnCl 2和 Et 3 N 副产物以及常用于钛 (III) 催化的盐酸盐添加剂的影响。我们进一步建议,对于 Cp 2 TiCl 2催化的反应,反应的加速阶段源于隐藏的自催化循环,这导致反应过程中的速率决定步骤发生变化。
更新日期:2021-09-17
中文翻译:

动力学分析揭示了钛 (III) 催化的酮-腈偶联物中隐藏的自催化和抑制途径
通过动力学分析,我们证明催化剂和底物储层的形成在钛 (III) 催化的酮腈偶联反应中起着至关重要的作用。结合先前研究的见解,组装了一个动力学模型,该模型反映了观察到的 ZnCl 2和 Et 3 N 副产物以及常用于钛 (III) 催化的盐酸盐添加剂的影响。我们进一步建议,对于 Cp 2 TiCl 2催化的反应,反应的加速阶段源于隐藏的自催化循环,这导致反应过程中的速率决定步骤发生变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号