当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Core–Shell Structured Cu(OH)2@NiFe(OH)x Nanotube Electrocatalysts for Methanol Oxidation Based Hydrogen Evolution
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2021-08-31 , DOI: 10.1021/acsanm.1c01236 Yue Liang 1 , Zhongxin Song 1 , Yan Zhang 1 , Bin Zhao 1 , Xuewan Wang 1 , Kun Xiang 1 , Zaochuan Ge 1 , Xian-Zhu Fu 1 , Jing-Li Luo 1
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2021-08-31 , DOI: 10.1021/acsanm.1c01236 Yue Liang 1 , Zhongxin Song 1 , Yan Zhang 1 , Bin Zhao 1 , Xuewan Wang 1 , Kun Xiang 1 , Zaochuan Ge 1 , Xian-Zhu Fu 1 , Jing-Li Luo 1
Affiliation
|
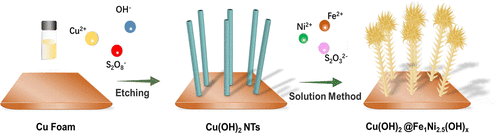
Electrochemical water splitting is considered to be a clean approach for hydrogen production. However, a large overpotential is required for the water oxidation reaction due to its sluggish kinetics, which leads to high energy consumption and low hydrogen production efficiency. Herein, copper hydroxide@nickel–iron hydroxide (Cu(OH)2@FeNi(OH)x) core–shell nanotube electrocatalysts are developed for an alternative methanol oxidation reaction to replace the conventional water oxidation reaction for boosting hydrogen evolution with less energy consumption. The average diameter of the Cu(OH)2@FeNi(OH)x nanotube is about 250 nm, and the surface is covered with FeNi(OH)x nanoflakes. The Cu(OH)2@FeNi(OH)x electrocatalysts demonstrate remarkable activity toward methanol oxidation, which require an anodic potential of only 1.32 V vs RHE to deliver a current density of 60 mA cm–2, being 160 mV lower than that of water oxidation. The lower potential of anodic reaction coupled with cathodic hydrogen evolution showcases the energy-saving capability in hydrogen production from water splitting. Moreover, methanol is exclusively converted into value-added formate with high selectivity and high Faradaic efficiency close to 100% in a wide potential range. The Cu(OH)2@FeNi(OH)x electrocatalysts also exhibit excellent stability for methanol–water coelectrolysis to produce pure hydrogen fuel and formate value-added chemicals.
中文翻译:

核壳结构的Cu(OH)2@NiFe(OH)x纳米管电催化剂用于甲醇氧化制氢
电化学水分解被认为是一种清洁的制氢方法。然而,由于其动力学缓慢,水氧化反应需要大的过电位,这导致高能耗和低产氢效率。在此,氢氧化铜@镍-氢氧化铁(Cu(OH) 2 @FeNi(OH) x)核-壳纳米管电催化剂被开发用于替代甲醇氧化反应,以取代传统的水氧化反应,以更少的能耗促进析氢. Cu(OH) 2 @FeNi(OH) x纳米管的平均直径约为250 nm,表面覆盖有FeNi(OH) x纳米薄片。Cu(OH) 2@FeNi(OH) x电催化剂对甲醇氧化表现出显着的活性,仅需要 1.32 V vs RHE 的阳极电位即可提供 60 mA cm –2的电流密度,比水氧化的电流密度低 160 mV。阳极反应的较低电位加上阴极析氢显示了水分解制氢的节能能力。此外,甲醇专门转化为具有高选择性和高法拉第效率在很宽的电位范围内接近 100% 的高附加值甲酸盐。Cu(OH) 2 @FeNi(OH) x电催化剂还表现出优异的甲醇-水共电解稳定性,以生产纯氢燃料和甲酸盐增值化学品。
更新日期:2021-09-24
中文翻译:

核壳结构的Cu(OH)2@NiFe(OH)x纳米管电催化剂用于甲醇氧化制氢
电化学水分解被认为是一种清洁的制氢方法。然而,由于其动力学缓慢,水氧化反应需要大的过电位,这导致高能耗和低产氢效率。在此,氢氧化铜@镍-氢氧化铁(Cu(OH) 2 @FeNi(OH) x)核-壳纳米管电催化剂被开发用于替代甲醇氧化反应,以取代传统的水氧化反应,以更少的能耗促进析氢. Cu(OH) 2 @FeNi(OH) x纳米管的平均直径约为250 nm,表面覆盖有FeNi(OH) x纳米薄片。Cu(OH) 2@FeNi(OH) x电催化剂对甲醇氧化表现出显着的活性,仅需要 1.32 V vs RHE 的阳极电位即可提供 60 mA cm –2的电流密度,比水氧化的电流密度低 160 mV。阳极反应的较低电位加上阴极析氢显示了水分解制氢的节能能力。此外,甲醇专门转化为具有高选择性和高法拉第效率在很宽的电位范围内接近 100% 的高附加值甲酸盐。Cu(OH) 2 @FeNi(OH) x电催化剂还表现出优异的甲醇-水共电解稳定性,以生产纯氢燃料和甲酸盐增值化学品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号