当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-Acetyl-l-cysteine-Loaded Nanosystems as a Promising Therapeutic Approach Toward the Eradication of Pseudomonas aeruginosa Biofilms
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2021-08-31 , DOI: 10.1021/acsami.1c05124 Rita M Pinto 1, 2, 3 , Claudia Monteiro 4 , Sofia A Costa Lima 1 , Susana Casal 1 , Patrick Van Dijck 2, 3 , M Cristina L Martins 4, 5 , Cláudia Nunes 1 , Salette Reis 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2021-08-31 , DOI: 10.1021/acsami.1c05124 Rita M Pinto 1, 2, 3 , Claudia Monteiro 4 , Sofia A Costa Lima 1 , Susana Casal 1 , Patrick Van Dijck 2, 3 , M Cristina L Martins 4, 5 , Cláudia Nunes 1 , Salette Reis 1
Affiliation
|
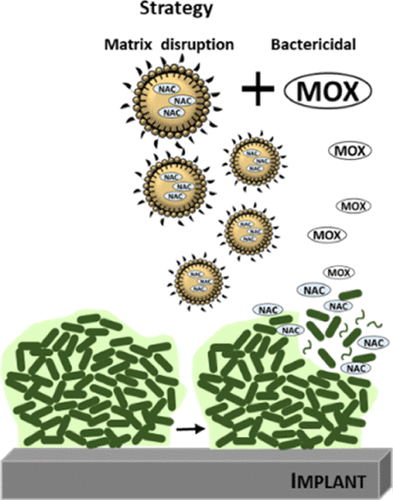
Bacterial biofilms are a major health concern, mainly due to their contribution to increased bacterial resistance to well-known antibiotics. The conventional treatment of biofilms represents a challenge, and frequently, eradication is not achieved with long-lasting administration of antibiotics. In this context, the present work proposes an innovative therapeutic approach that is focused on the encapsulation of N-acetyl-l-cysteine (NAC) into lipid nanoparticles (LNPs) functionalized with d-amino acids to target and disrupt bacterial biofilms. The optimized formulations presented a mean hydrodynamic diameter around 200 nm, a low polydispersity index, and a high loading capacity. These formulations were stable under storage conditions up to 6 months. In vitro biocompatibility studies showed a low cytotoxicity effect in fibroblasts and a low hemolytic activity in human red blood cells. Nevertheless, unloaded LNPs showed a higher hemolytic potential than NAC-loaded LNPs, which suggests a safer profile of the latter. The in vitro antibiofilm efficacy of the developed formulations was tested against Staphylococcus epidermidis (Gram-positive) and Pseudomonas aeruginosa (Gram-negative) mature biofilms. The results showed that the NAC-loaded LNPs were ineffective against S. epidermidis biofilms, while a significant reduction of biofilm biomass and bacterial viability in P. aeruginosa biofilms were observed. In a more complex therapeutic approach, the LNPs were further combined with moxifloxacin, revealing a beneficial effect between the LNPs and the antibiotic against P. aeruginosa biofilms. Both alone and in combination with moxifloxacin, unloaded and NAC-loaded LNPs functionalized with d-amino acids showed a great potential to reduce bacterial viability, with no significant differences in the presence or absence of NAC. However, the presence of NAC in NAC-loaded functionalized LNPs shows a safer profile than the unloaded LNPs, which is beneficial for an in vivo application. Overall, the developed formulations present a potential therapeutic approach against P. aeruginosa biofilms, alone or in combination with antibiotics.
中文翻译:

N-乙酰-l-半胱氨酸负载纳米系统作为根除铜绿假单胞菌生物膜的有希望的治疗方法
细菌生物膜是一个主要的健康问题,主要是因为它们有助于增加细菌对众所周知的抗生素的耐药性。生物膜的常规治疗是一项挑战,并且经常无法通过长期使用抗生素来实现根除。在这种情况下,目前的工作提出了一种创新的治疗方法,该方法专注于将N-乙酰-l-半胱氨酸 (NAC) 封装到用d-氨基酸功能化的脂质纳米颗粒 (LNP) 中,以靶向和破坏细菌生物膜。优化后的配方呈现出约 200 nm 的平均流体动力学直径、低多分散指数和高负载能力。这些制剂在长达 6 个月的储存条件下是稳定的。体外生物相容性研究表明,在成纤维细胞中具有低细胞毒性作用,在人类红细胞中具有低溶血活性。然而,未加载的 LNP 显示出比加载 NAC 的 LNP 更高的溶血潜力,这表明后者更安全。针对表皮葡萄球菌(革兰氏阳性)和铜绿假单胞菌(革兰氏阴性)成熟生物膜测试了所开发制剂的体外抗生物膜功效。结果表明,负载 NAC 的 LNP 对表皮葡萄球菌生物膜无效,而铜绿假单胞菌的生物膜生物量和细菌活力显着降低观察到生物膜。在更复杂的治疗方法中,LNPs 进一步与莫西沙星结合,揭示了 LNPs 和抗生素之间对铜绿假单胞菌生物膜的有益作用。无论是单独使用还是与莫西沙星组合,用d-氨基酸功能化的未加载和加载 NAC 的 LNP 都显示出降低细菌活力的巨大潜力,在 NAC 的存在或不存在方面没有显着差异。然而,NAC 负载的功能化 LNP 中 NAC 的存在显示出比未负载的 LNP 更安全的特性,这有利于体内应用。总体而言,开发的制剂提出了一种针对铜绿假单胞菌的潜在治疗方法 生物膜,单独或与抗生素联合使用。
更新日期:2021-09-15
中文翻译:

N-乙酰-l-半胱氨酸负载纳米系统作为根除铜绿假单胞菌生物膜的有希望的治疗方法
细菌生物膜是一个主要的健康问题,主要是因为它们有助于增加细菌对众所周知的抗生素的耐药性。生物膜的常规治疗是一项挑战,并且经常无法通过长期使用抗生素来实现根除。在这种情况下,目前的工作提出了一种创新的治疗方法,该方法专注于将N-乙酰-l-半胱氨酸 (NAC) 封装到用d-氨基酸功能化的脂质纳米颗粒 (LNP) 中,以靶向和破坏细菌生物膜。优化后的配方呈现出约 200 nm 的平均流体动力学直径、低多分散指数和高负载能力。这些制剂在长达 6 个月的储存条件下是稳定的。体外生物相容性研究表明,在成纤维细胞中具有低细胞毒性作用,在人类红细胞中具有低溶血活性。然而,未加载的 LNP 显示出比加载 NAC 的 LNP 更高的溶血潜力,这表明后者更安全。针对表皮葡萄球菌(革兰氏阳性)和铜绿假单胞菌(革兰氏阴性)成熟生物膜测试了所开发制剂的体外抗生物膜功效。结果表明,负载 NAC 的 LNP 对表皮葡萄球菌生物膜无效,而铜绿假单胞菌的生物膜生物量和细菌活力显着降低观察到生物膜。在更复杂的治疗方法中,LNPs 进一步与莫西沙星结合,揭示了 LNPs 和抗生素之间对铜绿假单胞菌生物膜的有益作用。无论是单独使用还是与莫西沙星组合,用d-氨基酸功能化的未加载和加载 NAC 的 LNP 都显示出降低细菌活力的巨大潜力,在 NAC 的存在或不存在方面没有显着差异。然而,NAC 负载的功能化 LNP 中 NAC 的存在显示出比未负载的 LNP 更安全的特性,这有利于体内应用。总体而言,开发的制剂提出了一种针对铜绿假单胞菌的潜在治疗方法 生物膜,单独或与抗生素联合使用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号