当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective α-Fluorination and α-Chlorination of N-Acyl Pyrazoles Catalyzed by a Non-C2-Symmetric Chiral-at-Rhodium Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-08-30 , DOI: 10.1021/acscatal.1c02901 Yvonne Grell 1 , Xiulan Xie 1 , Sergei I. Ivlev 1 , Eric Meggers 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-08-30 , DOI: 10.1021/acscatal.1c02901 Yvonne Grell 1 , Xiulan Xie 1 , Sergei I. Ivlev 1 , Eric Meggers 1
Affiliation
|
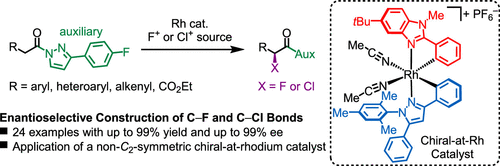
A non-C2-symmetric and sterically demanding chiral-at-rhodium catalyst is demonstrated to efficiently catalyze the highly enantioselective α-fluorination [12 examples, up to >99% enantiomeric excess (ee)] and α-chlorination (12 examples, up to 98% ee) of N-acyl pyrazoles in high yields. Based on two sterically distinct cyclometalating ligands, the nonracemic rhodium(III) catalyst can conveniently be accessed in an enantiomerically pure fashion (>99% ee) via an established auxiliary-mediated approach. Comparison of the catalytic performance with the related C2-symmetric rhodium catalysts revealed the explicit superiority of the non-C2-symmetric design for the presented α-halogenation reactions, which are generally featured by a very simple synthetic protocol.
中文翻译:

非C2对称手性铑催化剂催化N-酰基吡唑的对映选择性α-氟化和α-氯化
一种非C 2对称且空间要求高的铑手性催化剂被证明可有效催化高度对映选择性的 α-氟化 [12 个实例,高达 >99% 的对映体过量 (ee)] 和 α-氯化(12 个实例,高达 98% ee) 的N-酰基吡唑的高产率。基于两个空间上不同的环金属化配体,非外消旋铑 (III) 催化剂可以通过已建立的辅助介导方法以对映体纯的方式 (> 99% ee) 方便地获得。与相关C 2对称铑催化剂的催化性能比较揭示了非C 2催化剂的显着优势α-卤化反应的对称设计,通常具有非常简单的合成方案。
更新日期:2021-09-17
中文翻译:

非C2对称手性铑催化剂催化N-酰基吡唑的对映选择性α-氟化和α-氯化
一种非C 2对称且空间要求高的铑手性催化剂被证明可有效催化高度对映选择性的 α-氟化 [12 个实例,高达 >99% 的对映体过量 (ee)] 和 α-氯化(12 个实例,高达 98% ee) 的N-酰基吡唑的高产率。基于两个空间上不同的环金属化配体,非外消旋铑 (III) 催化剂可以通过已建立的辅助介导方法以对映体纯的方式 (> 99% ee) 方便地获得。与相关C 2对称铑催化剂的催化性能比较揭示了非C 2催化剂的显着优势α-卤化反应的对称设计,通常具有非常简单的合成方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号