当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remote Functionalization of 4-(Alk-1-en-1-yl)-3-Cyanocoumarins via the Asymmetric Organocatalytic 1,6-Addition
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-30 , DOI: 10.1002/adsc.202100660 Marta Romaniszyn 1 , Katarzyna Gronowska 1 , Łukasz Albrecht 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-30 , DOI: 10.1002/adsc.202100660 Marta Romaniszyn 1 , Katarzyna Gronowska 1 , Łukasz Albrecht 1
Affiliation
|
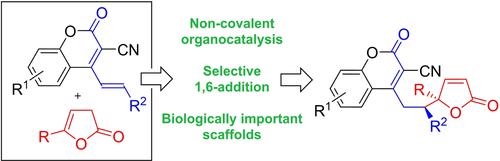
An organocatalytic 1,6-addition using 4-(alk-1-en-1-yl)-3-cyanocoumarins as acceptors has been developed. Dienolates derived from 5-substituted-furan-2(3H)-ones have been employed as pronucleophiles, therefore, enabling the synthesis of hybrid molecules bearing two biologically relevant units. Appropriate design of substrates and the application of quinine-derived catalyst resulted in very good site-selectivity as well as chemical and stereochemical efficiency of the process.
中文翻译:

通过不对称有机催化 1,6-加成对 4-(Alk-1-en-1-yl)-3-氰基香豆素进行远程官能化
已经开发了使用 4-(alk-1-en-1-yl)-3-cyanocoumarins 作为受体的有机催化 1,6-加成。源自 5-取代-呋喃-2(3 H )-酮的二烯醇化物已被用作亲核试剂,因此,能够合成带有两个生物学相关单元的杂化分子。适当的底物设计和奎宁衍生催化剂的应用导致该过程非常好的位点选择性以及化学和立体化学效率。
更新日期:2021-08-30
中文翻译:

通过不对称有机催化 1,6-加成对 4-(Alk-1-en-1-yl)-3-氰基香豆素进行远程官能化
已经开发了使用 4-(alk-1-en-1-yl)-3-cyanocoumarins 作为受体的有机催化 1,6-加成。源自 5-取代-呋喃-2(3 H )-酮的二烯醇化物已被用作亲核试剂,因此,能够合成带有两个生物学相关单元的杂化分子。适当的底物设计和奎宁衍生催化剂的应用导致该过程非常好的位点选择性以及化学和立体化学效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号