Structure ( IF 4.4 ) Pub Date : 2021-08-31 , DOI: 10.1016/j.str.2021.08.001 Zephan Melville 1 , Kookjoo Kim 1 , Oliver B Clarke 2 , Andrew R Marks 3
|
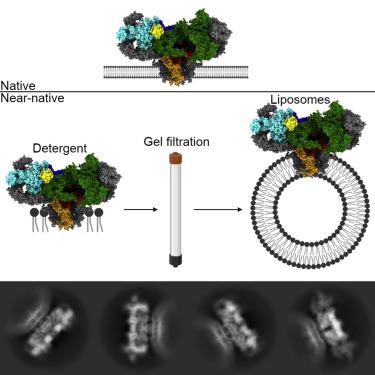
The type 1 ryanodine receptor (RyR)/calcium release channel on the sarcoplasmic reticulum (SR) is required for skeletal muscle excitation-contraction coupling and is the largest known ion channel, composed of four 565-kDa protomers. Cryogenic electron microscopy (cryo-EM) studies of the RyR have primarily used detergent to solubilize the channel; in the present study, we have used cryo-EM to solve high-resolution structures of the channel in liposomes using a gel-filtration approach with on-column detergent removal to form liposomes and incorporate the channel simultaneously. This allowed us to resolve the structure of the channel in the primed and open states at 3.4 and 4.0 Å, respectively, with a single dataset. This method offers validation for detergent-based structures of the RyR and offers a starting point for utilizing a chemical gradient mimicking the SR, where Ca2+ concentrations are millimolar in the lumen and nanomolar in the cytosol.
中文翻译:

膜嵌入骨骼肌兰尼碱受体的高分辨率结构
肌质网 (SR) 上的 1 型兰尼碱受体 (RyR)/钙释放通道是骨骼肌兴奋-收缩耦合所必需的,是已知最大的离子通道,由四个 565 kDa 的原体组成。RyR 的低温电子显微镜 (cryo-EM) 研究主要使用去污剂来溶解通道。在本研究中,我们使用冷冻电镜解决了脂质体中通道的高分辨率结构,使用凝胶过滤方法和柱上去垢剂去除形成脂质体并同时结合通道。这使我们能够使用单个数据集分别在 3.4 和 4.0 Å 处解析处于启动和打开状态的通道结构。2+浓度在管腔中为毫摩尔,在胞质溶胶中为纳摩尔。











































 京公网安备 11010802027423号
京公网安备 11010802027423号