当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Force-Dependent Interactions between Talin and Full-Length Vinculin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c06223 Yinan Wang 1 , Mingxi Yao 2 , Karen B Baker 3 , Rosemarie E Gough 3 , Shimin Le 1 , Benjamin T Goult 3 , Jie Yan 1, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c06223 Yinan Wang 1 , Mingxi Yao 2 , Karen B Baker 3 , Rosemarie E Gough 3 , Shimin Le 1 , Benjamin T Goult 3 , Jie Yan 1, 4
Affiliation
|
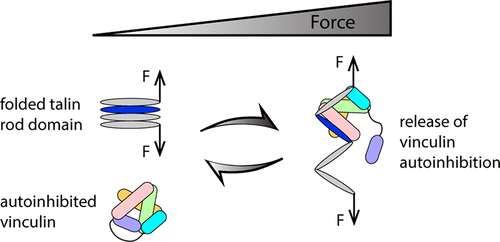
Talin and vinculin are part of a multicomponent system involved in mechanosensing in cell-matrix adhesions. Both exist in autoinhibited forms, and activation of vinculin requires binding to mechanically activated talin, yet how forces affect talin’s interaction with vinculin has not been investigated. Here by quantifying the kinetics of force-dependent talin–vinculin interactions using single-molecule analysis, we show that mechanical exposure of a single vinculin binding site (VBS) in talin is sufficient to relieve the autoinhibition of vinculin, resulting in high-affinity binding. We provide evidence that the vinculin undergoes dynamic fluctuations between an autoinhibited closed conformation and an open conformation that is stabilized upon binding to the VBS. Furthermore, we discover an additional level of regulation in which the mechanically exposed VBS binds vinculin significantly more tightly than the isolated VBS alone. Molecular dynamics simulations reveal the basis of this new regulatory mechanism, identifying a sensitive force-dependent change in the conformation of an exposed VBS that modulates binding. Together, these results provide a comprehensive understanding of how the interplay between force and autoinhibition provides exquisite complexity within this major mechanosensing axis.
中文翻译:

Talin 和全长 Vinculin 之间的力依赖相互作用
Talin 和 vinculin 是参与细胞-基质粘附中机械感应的多组分系统的一部分。两者都以自抑制形式存在,纽蛋白的激活需要与机械激活的 talin 结合,但尚未研究力如何影响 talin 与纽蛋白的相互作用。在这里,通过使用单分子分析量化依赖于力的 talin-vinculin 相互作用的动力学,我们表明 talin 中单个 vinculin 结合位点 (VBS) 的机械暴露足以减轻 vinculin 的自动抑制,从而导致高亲和力结合. 我们提供的证据表明纽蛋白在自动抑制的闭合构象和与 VBS 结合后稳定的开放构象之间发生动态波动。此外,我们发现了一个额外的调节水平,其中机械暴露的 VBS 与单独的 VBS 相比,与纽蛋白的结合更紧密。分子动力学模拟揭示了这种新调节机制的基础,确定了调节结合的暴露 VBS 构象的敏感力依赖变化。总之,这些结果提供了对力和自动抑制之间的相互作用如何在这个主要的机械传感轴内提供精细复杂性的全面理解。
更新日期:2021-09-15
中文翻译:

Talin 和全长 Vinculin 之间的力依赖相互作用
Talin 和 vinculin 是参与细胞-基质粘附中机械感应的多组分系统的一部分。两者都以自抑制形式存在,纽蛋白的激活需要与机械激活的 talin 结合,但尚未研究力如何影响 talin 与纽蛋白的相互作用。在这里,通过使用单分子分析量化依赖于力的 talin-vinculin 相互作用的动力学,我们表明 talin 中单个 vinculin 结合位点 (VBS) 的机械暴露足以减轻 vinculin 的自动抑制,从而导致高亲和力结合. 我们提供的证据表明纽蛋白在自动抑制的闭合构象和与 VBS 结合后稳定的开放构象之间发生动态波动。此外,我们发现了一个额外的调节水平,其中机械暴露的 VBS 与单独的 VBS 相比,与纽蛋白的结合更紧密。分子动力学模拟揭示了这种新调节机制的基础,确定了调节结合的暴露 VBS 构象的敏感力依赖变化。总之,这些结果提供了对力和自动抑制之间的相互作用如何在这个主要的机械传感轴内提供精细复杂性的全面理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号