当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reactivity of (bi-Oxazoline)organonickel Complexes and Revision of a Catalytic Mechanism
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c07139 Luchuan Ju 1 , Qiao Lin 1 , Nicole J LiBretto 2 , Clifton L Wagner 1 , Chunhua Tony Hu 1 , Jeffrey T Miller 2 , Tianning Diao 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-08-31 , DOI: 10.1021/jacs.1c07139 Luchuan Ju 1 , Qiao Lin 1 , Nicole J LiBretto 2 , Clifton L Wagner 1 , Chunhua Tony Hu 1 , Jeffrey T Miller 2 , Tianning Diao 1
Affiliation
|
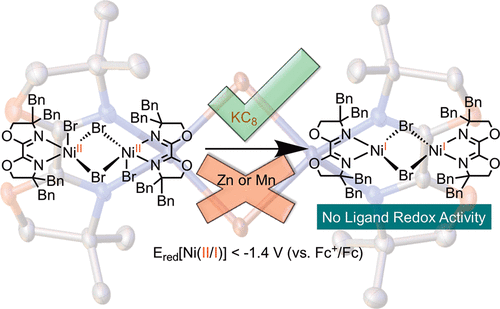
Bi-Oxazoline (biOx) has emerged as an effective ligand framework for promoting nickel-catalyzed cross-coupling, cross-electrophile coupling, and photoredox-nickel dual catalytic reactions. This report fills the knowledge gap of the organometallic reactivity of (biOx)Ni complexes, including catalyst reduction, oxidative electrophile activation, radical capture, and reductive elimination. The biOx ligand displays no redox activity in (biOx)Ni(I) complexes, in contrast to other chelating imine and oxazoline ligands. The lack of ligand redox activity results in more negative reduction potentials of (biOx)Ni(II) complexes and accounts for the inability of zinc and manganese to reduce (biOx)Ni(II) species. On the basis of these results, we revise the formerly proposed “sequential reduction” mechanism of a (biOx)Ni-catalyzed cross-electrophile coupling reaction by excluding catalyst reduction steps.
中文翻译:

(双恶唑啉)有机镍配合物的反应性和催化机理的修正
双恶唑啉 (biOx) 已成为促进镍催化交叉偶联、交叉亲电偶联和光氧化还原-镍双催化反应的有效配体框架。本报告填补了 (biOx)Ni 配合物的有机金属反应性的知识空白,包括催化剂还原、氧化亲电活化、自由基捕获和还原消除。与其他螯合亚胺和恶唑啉配体相比,biOx 配体在 (biOx)Ni(I) 配合物中不显示氧化还原活性。配体氧化还原活性的缺乏导致 (biOx)Ni(II) 配合物的负还原电位更大,并导致锌和锰无法还原 (biOx)Ni(II) 物种。基于这些结果,
更新日期:2021-09-15
中文翻译:

(双恶唑啉)有机镍配合物的反应性和催化机理的修正
双恶唑啉 (biOx) 已成为促进镍催化交叉偶联、交叉亲电偶联和光氧化还原-镍双催化反应的有效配体框架。本报告填补了 (biOx)Ni 配合物的有机金属反应性的知识空白,包括催化剂还原、氧化亲电活化、自由基捕获和还原消除。与其他螯合亚胺和恶唑啉配体相比,biOx 配体在 (biOx)Ni(I) 配合物中不显示氧化还原活性。配体氧化还原活性的缺乏导致 (biOx)Ni(II) 配合物的负还原电位更大,并导致锌和锰无法还原 (biOx)Ni(II) 物种。基于这些结果,



























 京公网安备 11010802027423号
京公网安备 11010802027423号