当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement and Correlation of Solubility Behavior of Bisphenol A in Binary (Ethyl Acetate + n-Butanol, Ethyl Acetate + 2-Butanol, Ethanol + n-Butanol, and Ethanol + 2-Butanol) Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-30 , DOI: 10.1021/acs.jced.1c00340 Ruina Fang 1, 2 , Yue Geng 1, 2 , Xinding Yao 1, 2 , Jingping Sun 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-30 , DOI: 10.1021/acs.jced.1c00340 Ruina Fang 1, 2 , Yue Geng 1, 2 , Xinding Yao 1, 2 , Jingping Sun 3
Affiliation
|
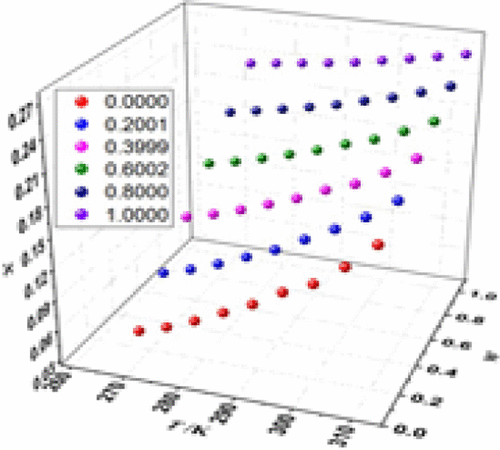
The solubility of bisphenol A in binary solvents (ethyl acetate + n-butanol, ethyl acetate + 2-butanol, ethanol + n-butanol, and ethanol + 2-butanol) was measured by a laser dynamic method under p = 101.1 kPa at temperatures ranging from 273.15 to 313.15 K. The solubility increased with increasing temperature at a given solvent composition; the solubility also increased with increasing content of ethyl acetate or ethanol at a given temperature. The modified Apelblat equation, λh equation, and Sun model were used to correlate the experimental solubility data; the values of RD and rmsd indicated that these models fitted the solubility data well.
中文翻译:

双酚 A 在二元(乙酸乙酯 + 正丁醇、乙酸乙酯 + 2-丁醇、乙醇 + 正丁醇和乙醇 + 2-丁醇)溶剂中溶解度行为的测量和相关性
双酚 A 在二元溶剂(乙酸乙酯 +正丁醇、乙酸乙酯 + 2-丁醇、乙醇 +正丁醇和乙醇 + 2-丁醇)中的溶解度在p = 101.1 kPa 温度下通过激光动力学方法测量范围从 273.15 到 313.15 K。在给定的溶剂组成下,溶解度随着温度的升高而增加;在给定温度下,溶解度也随着乙酸乙酯或乙醇含量的增加而增加。修正的 Apelblat 方程、λ h方程和 Sun 模型用于关联实验溶解度数据;RD 和 rmsd 的值表明这些模型很好地拟合了溶解度数据。
更新日期:2021-09-09
中文翻译:

双酚 A 在二元(乙酸乙酯 + 正丁醇、乙酸乙酯 + 2-丁醇、乙醇 + 正丁醇和乙醇 + 2-丁醇)溶剂中溶解度行为的测量和相关性
双酚 A 在二元溶剂(乙酸乙酯 +正丁醇、乙酸乙酯 + 2-丁醇、乙醇 +正丁醇和乙醇 + 2-丁醇)中的溶解度在p = 101.1 kPa 温度下通过激光动力学方法测量范围从 273.15 到 313.15 K。在给定的溶剂组成下,溶解度随着温度的升高而增加;在给定温度下,溶解度也随着乙酸乙酯或乙醇含量的增加而增加。修正的 Apelblat 方程、λ h方程和 Sun 模型用于关联实验溶解度数据;RD 和 rmsd 的值表明这些模型很好地拟合了溶解度数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号