当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of the powerful plasticity of the tert-butyl side chain on the conformational equilibrium of ascidiacyclamides
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-08-30 , DOI: 10.1002/psc.3363 Akiko Asano 1 , Maki Nakagawa 1 , Chihiro Miyajima 1 , Mami Yasui 1 , Katsuhiko Minoura 1 , Takeshi Yamada 1 , Mitsunobu Doi 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-08-30 , DOI: 10.1002/psc.3363 Akiko Asano 1 , Maki Nakagawa 1 , Chihiro Miyajima 1 , Mami Yasui 1 , Katsuhiko Minoura 1 , Takeshi Yamada 1 , Mitsunobu Doi 1
Affiliation
|
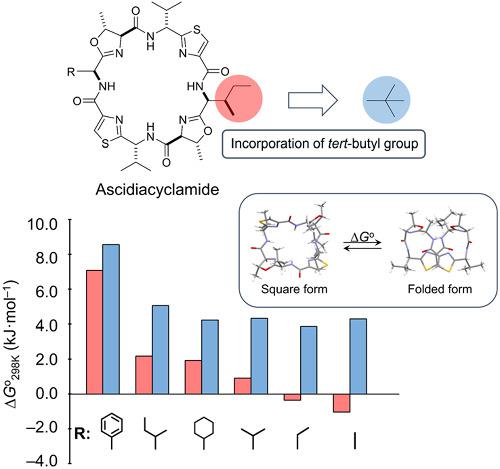
Ascidiacyclamide [cyclo(-Ile1,5-oxazoline2,6-d-Val3,7-thiazole4,8-)2] is a cytotoxic cyclic peptide from ascidian. Through structural analyses using monosubstituted analogues (Xaa1: Ala, 2-aminobutyric acid, Val, cyclohexylglycine, and phenylglycine), we previously demonstrated the conformational equilibrium between its square and folded forms. As the bulkiness of the Xaa1 residue side chain was reduced, spontaneous folding was promoted, and the cytotoxicity decreased accordingly. In the present study, five disubstituted analogues in which a tert-leucine residue (Tle) was incorporated at the 5-position of the abovementioned monosubstituted analogues were synthesized, after which their structures were analyzed using X-ray diffraction, circular dichroism (CD) spectral measurements, and 1H NMR-based quantitative analysis. The side chains of the Tle and Ile residues are structural isomers of one another, and the Tle residue bearing the tert-butyl group can be expected to play a role as a building block. In fact, peptides incorporating Tle5 exhibited much less spontaneous folding than their Ile5 counterparts in both crystal and solution. Increases in enthalpy and entropy due to the tert-butyl group during the folding process resulted in increased conformational free energy (ΔG°). The powerful plasticity of the tert-butyl group would stabilize the square form relating with cytotoxicity.
中文翻译:

叔丁基侧链的强塑性对水生环酰胺构象平衡的影响
Ascidiacyclamide [cyclo(-Ile 1,5 -oxazoline 2,6 - d -Val 3,7 -thiazole 4,8 -) 2 ] 是一种来自海鞘的细胞毒性环肽。通过使用单取代类似物(Xaa 1:Ala、2-氨基丁酸、Val、环己基甘氨酸和苯基甘氨酸)的结构分析,我们之前证明了其方形和折叠形式之间的构象平衡。随着Xaa 1残基侧链体积减小,自发折叠得到促进,细胞毒性相应降低。在本研究中,五个二取代的类似物,其中一个叔合成了在上述单取代类似物的5-位掺入-亮氨酸残基(Tle),然后使用X射线衍射、圆二色性(CD)光谱测量和基于1 H NMR的定量分析来分析它们的结构。Tle 和 Ile 残基的侧链是彼此的结构异构体,可以预期带有叔丁基的 Tle 残基起到结构单元的作用。事实上,结合 Tle 5的肽在晶体和溶液中表现出比 Ile 5对应物少得多的自发折叠。由于叔叔的焓和熵增加折叠过程中的-丁基导致构象自由能(ΔG °)增加。叔丁基的强大可塑性将稳定与细胞毒性相关的方形。
更新日期:2021-11-05
中文翻译:

叔丁基侧链的强塑性对水生环酰胺构象平衡的影响
Ascidiacyclamide [cyclo(-Ile 1,5 -oxazoline 2,6 - d -Val 3,7 -thiazole 4,8 -) 2 ] 是一种来自海鞘的细胞毒性环肽。通过使用单取代类似物(Xaa 1:Ala、2-氨基丁酸、Val、环己基甘氨酸和苯基甘氨酸)的结构分析,我们之前证明了其方形和折叠形式之间的构象平衡。随着Xaa 1残基侧链体积减小,自发折叠得到促进,细胞毒性相应降低。在本研究中,五个二取代的类似物,其中一个叔合成了在上述单取代类似物的5-位掺入-亮氨酸残基(Tle),然后使用X射线衍射、圆二色性(CD)光谱测量和基于1 H NMR的定量分析来分析它们的结构。Tle 和 Ile 残基的侧链是彼此的结构异构体,可以预期带有叔丁基的 Tle 残基起到结构单元的作用。事实上,结合 Tle 5的肽在晶体和溶液中表现出比 Ile 5对应物少得多的自发折叠。由于叔叔的焓和熵增加折叠过程中的-丁基导致构象自由能(ΔG °)增加。叔丁基的强大可塑性将稳定与细胞毒性相关的方形。









































 京公网安备 11010802027423号
京公网安备 11010802027423号