Redox Biology ( IF 10.7 ) Pub Date : 2021-08-31 , DOI: 10.1016/j.redox.2021.102120 Jian-An Pan 1 , Hui Zhang 2 , Hao Lin 1 , Lin Gao 1 , Hui-Li Zhang 1 , Jun-Feng Zhang 1 , Chang-Qian Wang 1 , Jun Gu 1
|
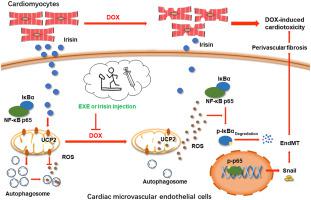
The dose-dependent toxicity to cardiomyocytes has been well recognized as a central characteristic of doxorubicin (DOX)-induced cardiotoxicity (DIC), however, the pathogenesis of DIC in the cardiac microenvironment remains elusive. Irisin is a new hormone-like myokine released into the circulation in response to exercise with distinct functions in regulating apoptosis, inflammation, and oxidative stress. Recent advances revealed the role of irisin as a novel therapeutic method and an important mediator of the beneficial effects of exercise in cardioprotection. Here, by using a low-dose long-term mouse DIC model, we found that the perivascular fibrosis was involved in its myocardial toxicity with the underlying mechanism of endothelial-to-mesenchymal transition (EndMT). Irisin treatment could partially reverse DOX-induced perivascular fibrosis and cardiotoxicity compared to endurance exercise. Mechanistically, DOX stimulation led to excessive accumulation of ROS, which activated the NF-κB-Snail pathway and resulted in EndMT. Besides, dysregulation of autophagy was also found in DOX-treated endothelial cells. Restoring autophagy flux could ameliorate EndMT and eliminate ROS. Irisin treatment significantly alleviated ROS accumulation, autophagy disorder, NF-κB-Snail pathway activation as well as the phenotype of EndMT by targeting uncoupling protein 2 (UCP2). Our results also initially found that irisin was mainly secreted by cardiomyocytes in the cardiac microenvironment, which was significantly reduced by DOX intervention, and had a protective effect on endothelial cells in a paracrine manner. In summary, our study indicated that DOX-induced ROS accumulation and autophagy disorders caused an EndMT in CMECs, which played a role in the perivascular fibrosis of DIC. Irisin treatment could partially reverse this phenomenon by regulating UCP2. Cardiomyocytes were the main source of irisin in the cardiac microenvironment. The current study provides a novel perspective elucidating the pathogenesis and the potential treatment of DIC.
中文翻译:

Irisin 通过调节内皮细胞中 ROS 积累和自噬紊乱抑制内皮间质转化,改善阿霉素诱导的心脏血管周围纤维化
对心肌细胞的剂量依赖性毒性已被广泛认为是阿霉素 (DOX) 诱导的心脏毒性 (DIC) 的核心特征,然而,心脏微环境中 DIC 的发病机制仍不清楚。鸢尾素是一种新的激素样肌因子,响应运动而释放到循环中,在调节细胞凋亡、炎症和氧化应激方面具有独特的功能。最近的进展揭示了鸢尾素作为一种新型治疗方法的作用以及运动在心脏保护方面的有益作用的重要介质。在这里,通过使用低剂量长期小鼠 DIC 模型,我们发现血管周围纤维化与其心肌毒性有关,其潜在机制是内皮间质转化(EndMT)。与耐力运动相比,鸢尾素治疗可以部分逆转阿霉素诱导的血管周围纤维化和心脏毒性。从机制上讲,DOX 刺激导致 ROS 过度积累,从而激活 NF-κB-Snail 通路并导致 EndMT。此外,在 DOX 处理的内皮细胞中也发现自噬失调。恢复自噬通量可以改善 EndMT 并消除 ROS。Irisin 治疗通过靶向解偶联蛋白 2 (UCP2) 显着减轻 ROS 积累、自噬紊乱、NF-κB-Snail 通路激活以及 EndMT 表型。我们的研究结果还初步发现,心脏微环境中鸢尾素主要由心肌细胞分泌,通过DOX干预显着减少,并以旁分泌方式对内皮细胞产生保护作用。总之,我们的研究表明,DOX 诱导的 ROS 积累和自噬紊乱导致 CMEC 中的 EndMT,从而在 DIC 血管周围纤维化中发挥作用。鸢尾素治疗可以通过调节 UCP2 部分逆转这种现象。心肌细胞是心脏微环境中鸢尾素的主要来源。目前的研究为阐明 DIC 的发病机制和潜在治疗提供了新的视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号